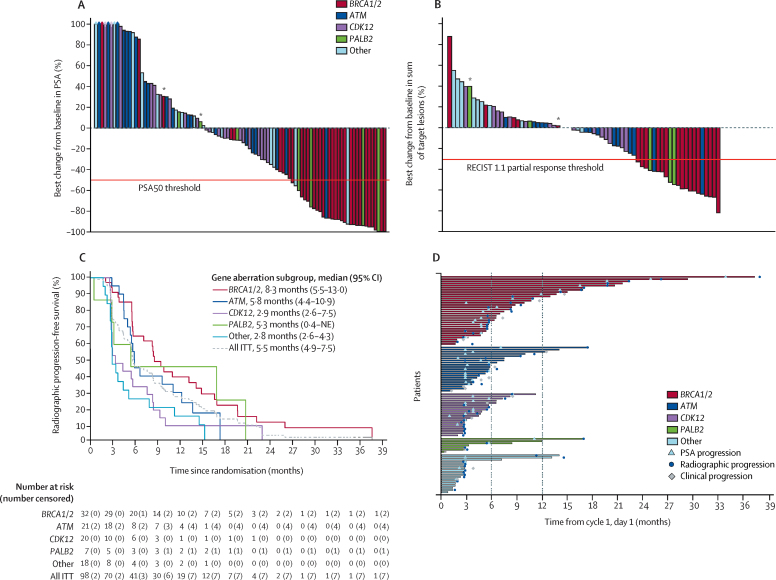
Figure 3.
Antitumour activity by gene aberration subgroup (intention-to-treat population, pooled 300 mg and 400 mg cohorts)
(A) Maximum percentage change from baseline in PSA during treatment. (B) Maximum percentage change from baseline in the sum of target lesions (Response Evaluation Criteria in Solid Tumors 1·1) during treatment. (C) Radiographic progression-free survival. (D) Swimmers plot of time on treatment for each patient. ITT=intention-to-treat. NE=not estimable. PSA=prostate-specific antigen. PSA50=decrease in prostate-specific antigen of ≥50%. *Patients presenting with mutliple mutations are represented in a single subgroup.