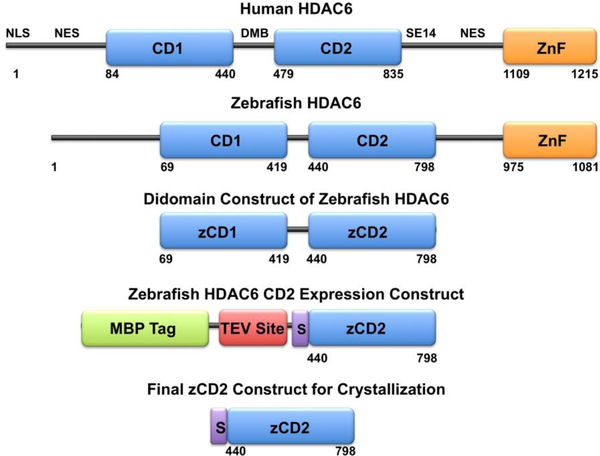
Figure 1.
The primary structure of full-length human (Homo sapiens) HDAC6 reveals domain organization as follows: NLS, nuclear localization signal; NES, nuclear export signal; CD1, catalytic domain 1; DMB, dynein motor binding domain; CD2, catalytic domain 2; SE14, Ser-Glu tetradecapeptide repeat; ZnF, zinc-finger domain. Catalytic domain 2 is the tubulin deacetylase domain. The primary structure of full-length zebrafish (Danio rerio) HDAC6 reveals similar domain organization. Truncation of the zebrafish enzyme to a CD1-CD2 construct, followed by the substitution of CD1 with a maltose binding protein (MBP) fusion tag along with a tobacco etch virus (TEV) protease cleavage site, yields the final expression construct with the additional peptide segment SNAGG (S) at the N-terminus of zCD2. Cleavage with TEV protease yields the final zCD2 construct used for assay and crystallization.