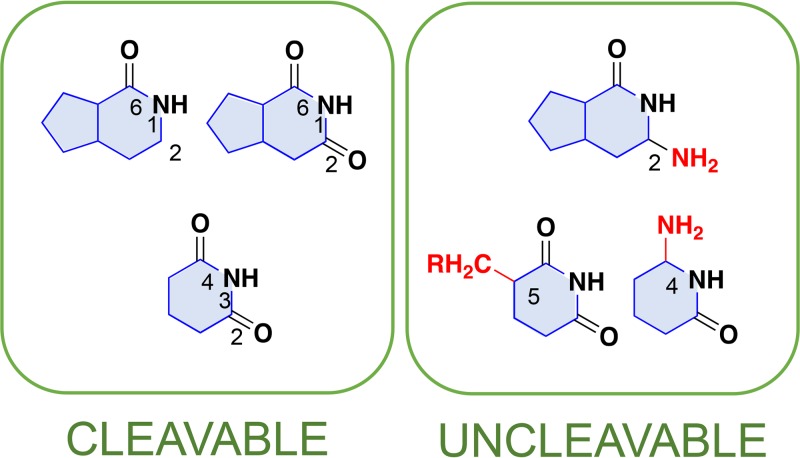
FIG 6.
Nucleobase structures that are cleavable/uncleavable by PfuEndoQ. The features in nucleobases, which are cleavable and uncleavable by PfuEndoQ, are summarized. The favorable structure for the EndoQ activity is the imide structure in C-2, N-1, and C-6 in purine bases (C-2: “=CO” or “≡CH”) and C-2, N-3, and C-4 in pyrimidine bases. Groups shown in red might cause the inhibition of the EndoQ activity as follows: C-4 amino group and C-5 methylation of pyrimidine, and C-2 amino group in purine.