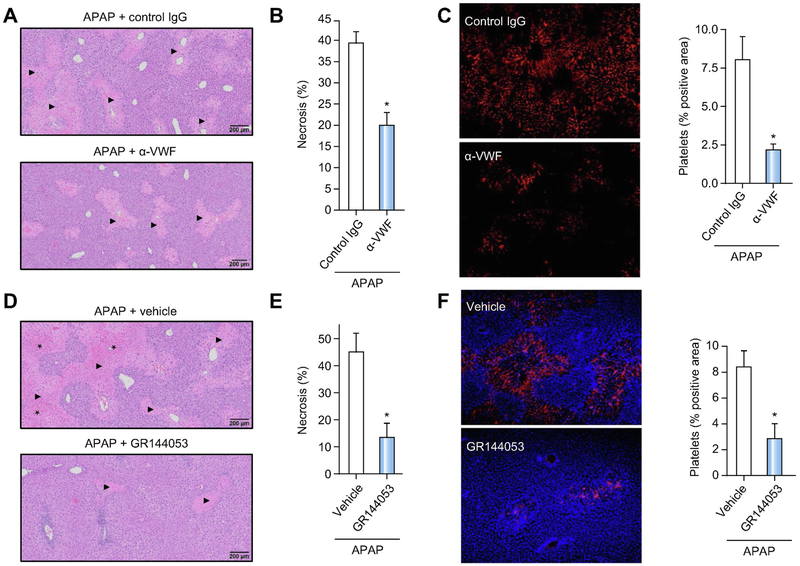
Figure 7. Blocking VWF function or platelet integrin αIIbβ3 accelerates liver repair after APAP-induced liver injury in mice.
For A-C, APAP-challenged wild-type mice were treated with a polyclonal rabbit anti-human VWF antibody (α-VWF, i.p. 50 μg/mouse) or control IgG,4h and 24h after APAP administration and samples were collected 48h after APAP challenge. (A) Representative photomicrographs of H&E-stained liver sections depict hepatocellular necrosis (closed triangles). (B) Area of hepatocellular necrosis. (C) Representative images showing immunolabeling of CD41 (platelets; red) in liver sections from control mice (upper left panel) or α-VWF treated mice (lower left panel) and quantification of platelet deposition in control-treated (black bars) mice and α-VWF treated (grey bars) mice (right panel), expressed as percentage of positive pixel count. For D-F, APAP-challenged wild-type mice were treated with a platelet αIIbβ3 antagonist (GR144053, i.p. 10mg/kg) or vehicle (saline), 12h after APAP administration and samples were collected 48h after APAP challenge. (D) Representative photomicrographs of H&E-stained liver sections depict hepatocellular necrosis (closed triangles) and congestion/hemorrhage (asterisk). (E) Area of hepatocellular necrosis. (F) Representative images showing immunolabeling of CD41 (platelets; red) in DAPI (blue)-counterstained liver sections from vehicle mice (upper left panel) or GR144053-treated mice (lower left panel), and quantification of platelet deposition in vehicle-treated (black bar) mice and GR144053-treated (grey bar) mice (right panel), expressed as percentage of positive pixel count. Bars represent mean + standard error of the mean (n = 5-10 mice per group). * p<0.05 compared to vehicle/control antibody-treated mice.