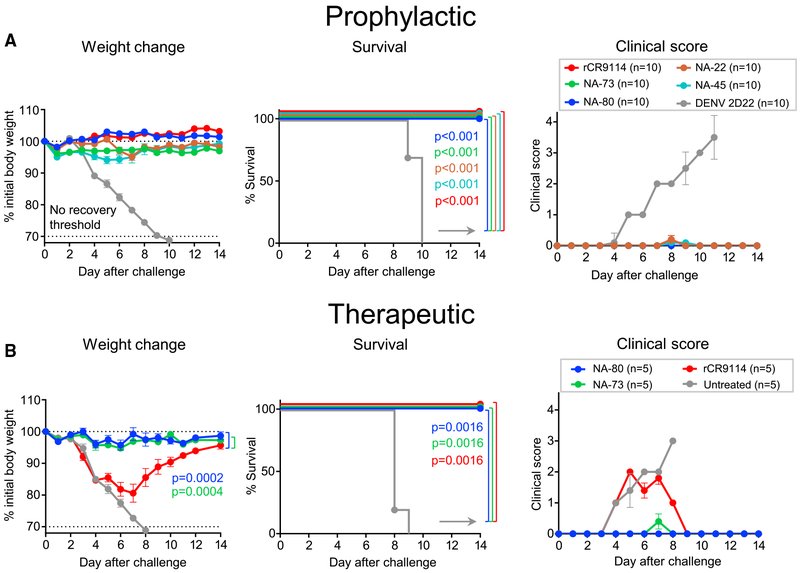
Figure 6. Anti-N9 mAbs Mediate a High Level of Protection In Vivo against Lethal H7N9 Challenge in Mice.
Groups of BALB/c mice were inoculated intraperitoneally (i.p.) one day before (A) or one day after (B) virus challenge with 10 mg/kg of anti-N9 mAb or with DENV 2D22 control mAb reactive to an irrelevant antigen (dengue virus) or with 10 mg/kg of a recombinant mAb on the basis of the sequence of the broadly neutralizing influenza stem-targeted mAb rCR9114. On day 0 (d0), mice were challenged intranasally (i.n.) with a lethal dose of influenza A/Shanghai/02/2013 IDCDC-RG32A virus and monitored for protection. The weights and clinical scores are represented as the group mean ± SEM. The lower dotted line indicates the no-recovery threshold (> 30% weight loss) and endpoint for euthanasia. Body weight change curves in (B) were compared by overall test using two-way ANOVA. Survival curves were estimated using the Kaplan-Meier method. Survival of each group that was treated with an anti-NA or anti-HA (rCR9114) mAb was compared with the control group as indicated using log rank (Mantel-Cox) test. A clinical score of 4 corresponds to a moribund state. Data represent one experiment, and “n” symbol in the plots indicates number of mice per group for each treatment condition (5–10 mice per group).