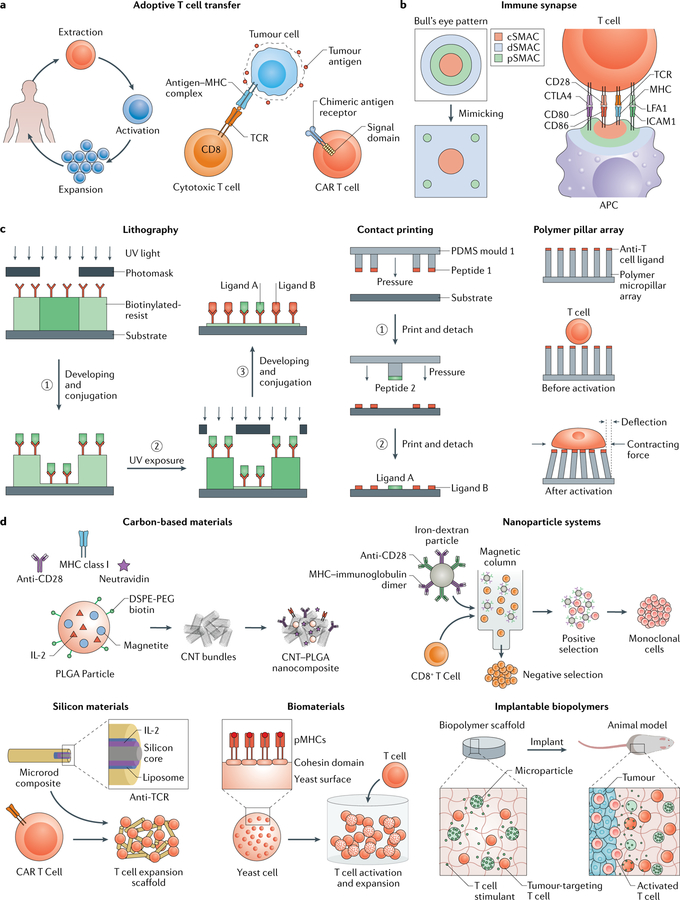
Fig. 4 |. Engineering activated T cells.
a | For adoptive T cell transfer, T cell receptors (TCRs) can be modified ex vivo to make them specific for disease-causing cells by incubation with disease-causing cells (through an interaction between TCR and antigen bound to a major histocompatibility complex (MHC)) or by inducing expression of a chimeric antigen receptor (CAR), which includes a specified antigen-binding domain directly linked to downstream signalling domains, b | The immune synapse between a T cell and an antigen-presenting cell (APC) has a bull’s eye pattern, with a central supramolecular activation cluster (cSMAC), a peripheral SMAC (pSMAC) and a distal SMAC (dSMAC). This structure can be mimicked using 2D substrates, c | 2D approaches, such as lithography, contact printing and polymer pillar arrays, can be applied to study immune synapse organization and mechanosignalling pathways. In lithography, a biotinylated photoresist that dissolves in aqueous buffers upon UV exposure enables control of protein presentation through iterative UV exposure, dissolution and conjugation steps. Microcontact printing can be used to functionalize a substrate with multiple ligands by applying repeated rounds of contact between several coated polydimethylsiloxane (PDMS) stamps and the substrate. Micropillar arrays modified with anti-T cell ligands can be applied to measure mechanical forces during T cell activation on the basis of the dimension, composition and deflection of the pillars, d | 3D approaches can be applied to develop artificial APCs for T cell activation and expansion. Carbon nanotubes can be functionalized with neutravidin, which is bound to biotinylated anti-CD28 antibodies, MHC class I and poly(lactide-co-glycolide) (PLGA) nanoparticles encapsulating interleukin-2 (IL-2). Paramagnetic iron-dextran nanoparticles can be functionalized with anti-CD28 and MHC to select and expand specific T cells. Mesoporous silica microrods can be functionalized with IL-2 and coated with biotinylated liposomes bound to peptide-bound MHC, anti-CD3 and anti-CD28 to design a modular APC-mimetic scaffold, for example, for CAR T cell expansion. Yeast cells can be engineered to display cohesion domains that bind to dockerin-fused proteins for the design of protein scaffolds. The yeast-based scaffolds can be used to control the stoichiometric and spatial organization of T cell ligands. APC-mimicking and T cell-stimulating microparticles can be incorporated into implantable and degradable biomaterials to deliver T cell therapies to the tumour microenvironment. CNT, carbon nanotube; DSPE-PEG, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-(amino(polyethylene glycol)); ICAM1, intercellular adhesion molecule 1; LFA1, lymphocyte function-associated antigen 1; pMHC, peptide-MHC.