Summary
We study the economics and energy efficiency of biorefineries employing lignin valorization. We use superstructure-based process synthesis to study different configurations under different types of constraints. Using optimization, we examine the impact of various parameters for lignin valorization such as bioproduct selling price, production cost, conversion coefficient, and energy requirement. The results show that the optimal strategy leading to a minimum ethanol selling price (MESP) of $3.44/GGE does not include lignin valorization. Results indicate that under certain scenarios, the optimal biorefinery strategies with lignin valorization tend to be energy deficient, and thus the optimal pretreatment technology may switch from γ-valerolactone-based deconstruction to ammonia fiber expansion. Further analysis is performed to study how improvements in combinations of selected parameters can lead to lower cost for a thermal-neural biorefinery.
Subject Areas: Bioengineering, Industrial Engineering, Systems Engineering, Bioresources, Metabolic Engineering
Graphical Abstract
Highlights
-
•
Developed optimization model to evaluate biorefinery with lignin valorization
-
•
Studied impact of technical, economic, and energy parameters on economics
-
•
Lignin utilization is optimized to achieve a thermal-neutral biorefinery
-
•
Established technology targets for economic and energetic feasibility
Bioengineering; Industrial Engineering; Systems Engineering; Bioresources; Metabolic Engineering
Introduction
Fossil-based fuels and chemicals have provided the majority of energy needed for human development over the last century (U.S. Energy Information Administration, 2018). However, with the rising concerns over global warming and depleting fossil resources, the global community has come to the conclusion that a switch to renewable sources is critical to sustain future developments (UNGA, 2015). In US, a potential biomass availability of more than 1 billion dry tons per year is projected by 2030 providing a large resource for renewable energy (Langholtz et al., 2016). Lignocellulosic biomass is of particular interest as it can be collected in large quantities from agricultural and forestry resources and is inedible and carbon neutral, avoiding food vs fuel issues associated with grain and crop based biomass (Sun et al., 2018).
Lignocellulosic biomass has three major constituents: cellulose, hemicellulose, and lignin (Mussatto, 2016). Cellulose (30–50 wt%) is a linear chain polysaccharide consisting of hundreds to thousands of β (1→4)-linked D-glucose molecules (Upton and Kasko, 2016). Hemicellulose (20–35 wt%) is an amorphous heteropolymer of primarily xylose sugars (Gibson, 2012). Lignin (15–30 wt%) is a complex aromatic heteropolymer derived from three cinnamyl alcohol monomers, i.e., p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol linked by carbon-carbon and ether bonds producing, respectively, p-hydroxyphenyl (H), guaiacyl (G), and syringyl phenylpropanoid (S) units in lignin polymer (Boerjan et al., 2003, Ralph et al., 2019). To develop economical and sustainable biorefineries, all three major constituents must be effectively converted to value-added products. Over the last decades, significant progress has been made in cellulose and hemicellulose deconstruction and monomer upgrading technologies to fuels and chemicals (Chandel et al., 2018, Langan et al., 2011, Op de Beeck et al., 2015, Peng et al., 2012, Zabed et al., 2016). In contrast, lignin valorization technologies have been limited, for the most part, to heat and power production (Da Costa Sousa et al., 2016, Vardon et al., 2015).
Native lignin is covalently cross-linked with hemicellulose, which together form an amorphous structure to enclose and protect cellulose fibers from microbial attack (Schutyser et al., 2018). Biomass deconstruction is performed to breakdown the covalent bonds with holocellulose (cellulose and hemicellulose) and yield isolated lignin prior to valorization. Generally, fractionation strategies can be divided into two categories: (1) methods resulting in lignin release from the biomass called delignification and (2) methods targeting conversion and solubilization of carbohydrates. Delignification processes include alkaline, acidic, reductive, ionic liquid dissolution, and mechanical pretreatment followed by extraction, which produce lignin precipitate or depolymerized lignin oil. The second category includes acid-catalyzed hydrolysis, enzyme-assisted hydrolysis, and thermal processes, which isolate lignin in the form of precipitate or insoluble residue. See Schutyser et al. (Schutyser et al., 2018) for a detailed review of each category.
Native lignin polymer contains carbon-carbon and ether inter-unit connections (Boerjan et al., 2003, Vanholme et al., 2010). The most abundant linkage bond (>50%) is β-O-4 alkyl-aryl ether linkage, which, along with α-O-4 ether linkage, is the most easily cleavable bond (Chakar and Ragauskas, 2004). Considering that carbon-carbon bonds are difficult to break, the majority of lignin depolymerization strategies target the ether bonds (Gall et al., 2017, Rahimi et al., 2014). Generally, the theoretical monomer yield is proportional to the square of the relative content of inter-unit ether bonds (Yan et al., 2008). Therefore, to achieve higher depolymerization yields, the ether bonds must remain intact during biomass fractionation. The monomer yield from lignin strongly depends on three major factors: (1) isolation strategy, (2) depolymerization strategy, and (3) lignin origin (biomass type) (Schutyser et al., 2018). Lignin depolymerization strategies could be categorized to (1) reductive, (2) oxidative, (3) base- and acid-catalyzed, (4) solvolytic and thermal, and (5) two-step depolymerization (Schutyser et al., 2018). Table 1 provides a summary of each category. For a more detailed discussion, readers are referred to reviews of this topic (Behling et al., 2016, Li et al., 2015, Ma et al., 2015, Mu et al., 2013, Schutyser et al., 2018, Xiu and Shahbazi, 2012, Zakzeski et al., 2010).
Table 1.
Summary and Characteristics of Lignin Depolymerization Strategies
| Depolymerization | Catalyst | Additives | Solvents | T (°C) | PH2 (bar) | Selectivity | Yield |
|---|---|---|---|---|---|---|---|
| Reductive | |||||||
| Mild hydroprocessing (MHD) | Nobel metal, base metal, mixed metal | H3PO4, HCl, MClx, NaOH, KOH, Na2CO3 | H2O, MeOH, EtOH, iPrOH, dioxane, tetrahydrofuran, or solvent mixture | 130–390 | 10–100 | High toward methoxyphenols or catechols | Moderate <20wt% |
| Harsh hydroprocessing (HHD) | Nobel metal, base metal | Mostly solventless, MeOH, 1-methylnaphthalene | 320–450 | 35–100 | Low toward phenol, methylated phenols, and phenols with long alkyl chains | Moderate <30wt% |
|
| Bifunctional hydroprocessing (BHD) | Nobel metal, base metal | H2O, MeOH, tetrahydrofuran, heptane, methyl cyclohexane, dodecane, hexadecane | 150–320 | 20–70 | High toward cycloalkanes C6–C18 | High <50%–70% |
|
| Liquid phase reforming (LPRD) | Nobel metal, base metal | H-zeolites, nafion SAC-13, H3PO4, heteropolyacid, NaOH | H2O, formic acid, MeOH, EtOH, iPrOH, tetralin, glycerol | 150–400 | Liquid phase | Very low toward a broad range of compounds | High 20%–86% |
| Depolymerization | Catalyst | Oxidants | Solvents | T (°C) | PO2 (bar) | Selectivity | Yield |
|---|---|---|---|---|---|---|---|
| Oxidative | |||||||
| Alkaline oxidation to phenolics (AlOF) | Soluble catalyst, solid catalyst | O2 | NaOH in H2O, MeOH, EtOH, dioxane, tetrahydrofuran, KOH in water | 120–190 | 2–14 | High toward phenolic aldehydes such as vanillin | Low <10%–20% |
| Acidic (AcOF) and pH-neutral (NOF) lignin oxidation to phenolics | Soluble catalyst, solid catalyst | O2, H2O2, peracetic acid | H2O, MeOH, acetic acid, methyl isobutyl ketone, ionic liquid | 60–210 | 5–30 | Moderate toward phenols | Low <10–20% |
| Lignin oxidation to non-phenolic carboxylic acids (OCA) | Solid catalyst | O2, H2O2 | Mainly liquid phase H2O (neutral), acidic (H2SO4 or acetate buffer), alkaline (NaOH) | 60–225 (liquid phase), 327–377 (gas phase) |
High toward carboxylic acids (formic, acetic, succinic, oxalic, and malonic acids) | High 10%–60% |
|
| Depolymerization | Catalyst | Additives | Solvents | T (°C) | Selectivity | Yield |
|---|---|---|---|---|---|---|
| Base- & Acid-Catalyzed | ||||||
| Base-catalyzed depolymerization (BCD) | Soluble base (mostly NaOH) or solid base | H2O (mostly), MeOH, EtOH, iPrOH, tetrahydrofuran, 3-methyl-3-pentanol | 240–330 | Moderate, methoxyphenols (T < 300), catechol (T > 300) | <10%–20% | |
| Acid-catalyzed depolymerization (ACD) | Lewis acid, solid or soluble Brønsted acid | [Ir(cod)Cl]2/PPh3, [Rh(cod)Cl]2/dppp | H2O, MeOH, EtOH, iPrOH, 1-BuOH, ethylene glycol, dioxane, octane, formic acid | 140–400 | Low with wide array of products, methoxyphenols (T < 300), catechol (T > 300) | <20%–60% |
| Depolymerization | Catalyst | Solvents | T (°C) | Selectivity | Yield | Note |
|---|---|---|---|---|---|---|
| Solvolytic & Thermal | ||||||
| Solvolytic (SLD) | Water, MeOH, EtOH, iPrOH, 1-BuOH, tetrahydrofuran, acetone, octane, dihydroanthracene, tetralin, naphthalene, solvent mixture | 250–450 | Low | <10%–20% | Hydrogen-donating solvents are mostly used | |
| Fast pyrolysis (FPD) | 400–800 | Low | <20% | Under inert atmosphere | ||
| Catalytic fast pyrolysis (CFP) | In situ or ex situ: silica/alumina, zeolites, metal on zeolite oxides | 500–700 | High toward deoxygenated aromatics, benzene, toluene, xylene, and naphthalene | <30% | Under inert atmosphere | |
| Depolymerization | Catalyst 1st Stage | Catalyst 2nd Stage | T (°C) | Selectivity | Yield | Note |
|---|---|---|---|---|---|---|
| Two-Stage | ||||||
| Benzylic alcohol oxidation and depolymerization (BAOD) | 4-acetoamide-TEMPO/HNO3/HCl, DDQ/tBuONO, [4-AcNH-TEMPO]BF4-mediated, NHPI/2,6-lutidine-mediated | Aqueous formic acid/sodium formate | 110 | Phenolic diketones (syringyl- and guaiacyl-1,2-propanedione), aldehydes (syringaldehyde and vanillin), and acids (syringic, vanillic, and p-hydroxybenzoic acid) | 52% | Under aerobic oxidation or electrocatalytic oxidation |
| Benzylic alcohol methylation and depolymerization (BAMD) | Al2(SO4)3 | Pd/C, liquid phase reforming in methanol/formic acid | 280 | Low | 17% | Under microwave radiation and methanol solvent |
Some of the lignin depolymerization monomers such as vanillin can be sold without further transformation. However, many other monomer streams require additional processing to narrow the wide range of monomers into targeted categories to (1) favor economical separation and recovery and (2) produce marketable products. Generally, monomer upgrading can be accomplished through biocatalytic or chemocatalytic processes.
In biocatalytic processes, depolymerized lignin is transformed to value-added chemicals mostly using aerobic microbial organisms (Schutyser et al., 2018). Microorganisms could be genetically engineered to defunctionalize aromatic compounds into intermediate products such as gallate, protocatechuate, and catechol. Dioxygenase enzymes could further ring-open the intermediate products and utilize them in central carbon metabolism (Abdelaziz et al., 2016, Masai et al., 2007, Masai et al., 2012). Metabolic engineering has enabled the use of microorganisms for the production of chemicals such as vanillin (Varman et al., 2016), medium-chain-length polyhydroxyalkanoates (Linger et al., 2014), muconic acid (Vardon et al., 2015), pyridine dicarboxylic acids (Mycroft et al., 2015, Perez et al., 2019), and fatty acids (Zhao et al., 2016), which could be further upgraded to high-value chemicals and polymer building blocks.
Chemocatalytic upgrading reactions can be divided into two categories: (1) reactions that transform phenolic core and its substitution degree and (2) reactions that target structure of side-chains (Schutyser et al., 2018). In phenolic core transformation, defunctionalization of core phenol is targeted using hydrodeoxygenation (HDO) reactions to reduce the complexity, functionality, H/C, and O/C ratio in depolymerization products. Accordingly, the HDO reactions could target production of four types of products: (1) alkenes, (2) aromatics, (3) phenols, and (4) cyclohexanols, which are different in oxygen content (depending on partial or complete HDO) and aromaticity (ring hydrogenation or preservation). Table 2 provides a summary of characteristics and operating conditions of monomer upgrading processes. More detailed reviews of upgrading processes are available in the literature (Abdelaziz et al., 2016, Beckham et al., 2016, Bugg and Rahmanpour, 2015, Laskar et al., 2013, Li et al., 2015, Zakzeski et al., 2010).
Table 2.
Summary and Characteristics of Upgrading Strategies for Lignin-Derived Monomers
| Upgrading Process | Target Products | Product Value | C-H-O Ratio | Catalyst | Note |
|---|---|---|---|---|---|
| Chemocatalytic | Alkanes and cyclohexanes | Low-value, mid-range fuel additive | High H/C, low O/C | Noble metals (Ru, Rh, Pd, Pt), Ni-based catalyst, H3PO4, acetic acid, acidic IL, HZSM-5, HBEA | Monomers are ring opened and products are fully deoxygenated |
| Aromatic hydrocarbons | Low value, mid-range fuel additive | Low H/C, low O/C | Co-Mo, NiMo, MoO3, FeMoP, Ru/TiO2, PdFe/C, PtCo/C | Operated at gas phase, high temperature, and low H2 pressure (<1 bar) for CO hydrogenation. Products are fully deoxygenated | |
| Cyclohexanols | As feed for synthesis of high-value monomers (e.g., adipic acid and polyester building blocks) | High H/C, high O/C | Ni/CeO2, Ni/SiO2–Al2O3, RANEYs Ni, CoNx/C,Ru/ZrO2–La(OH)3, Ru–MnOx/C, and Ru/C + MgO | Operated in liquid phase, partial HDO, demethoxylation, and aromatic ring hydrogenation | |
| Phenols | As feed for synthesis of high-value monomers (e.g., terephthalic acid, ethylene, propylene, and phenol) | Low H/C, high O/C | Nobel metal, base metal, | Selective demethoxylation | |
| Biological | Vanillin, medium-chain-length polyhydroxyalkanoates, muconic acid | Precursor to adipic acid, terephthalic acid, pyridine dicarboxylic acids, and fatty acids | Close to theoretical yields obtained from representative components such as p-coumarate, ferulate, and benzoate |
In summary, lignin is the second most abundant natural polymer accounting for about 30% of organic carbon in the biosphere and is the largest renewable source of aromatic monomers (Li et al., 2018, Stolark, 2017). The polyphenolic structure of lignin can potentially be used to produce value-added chemicals, functional materials, and fuel products (Schutyser et al., 2018, Zhang et al., 2017). However, being at early stage of development, the economic viability of lignin valorization strategies and their integration within biorefineries are not known.
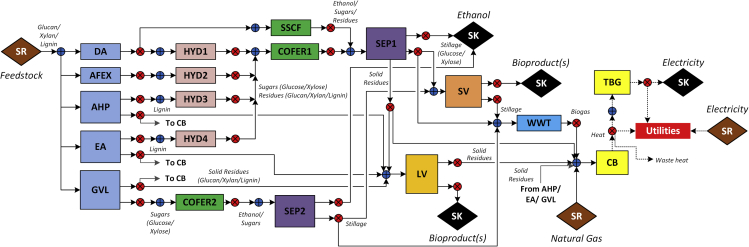
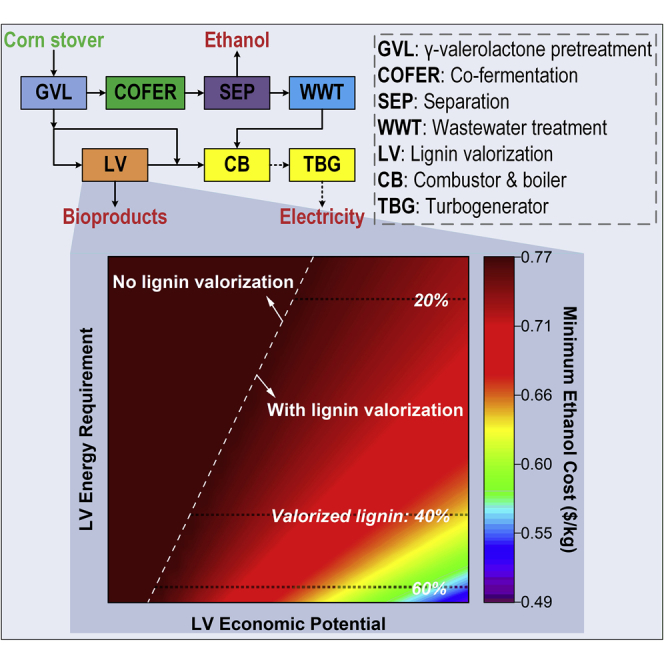
Accordingly, the goal of this paper is to study what technological advances are necessary for lignin valorization technologies to become attractive or, equivalently, what is the impact of uncertainty in some key lignin-valorization-related technological and economic parameters on the viability of lignin valorization strategies. Toward this goal, we first generate a superstructure to represent potential biorefinery configurations (Figure 1) and then a mixed-integer nonlinear programming model (see Methods for details) to identify the optimal process to achieve specific objectives while satisfying given constraints. Using this optimization model, we then evaluate the impact of four critical lignin valorization parameters on the energy efficiency and economics: (1) energy requirement of conversion, (2) conversion efficiency to bioproducts, (3) production cost, and (4) market value of bioproducts.
Figure 1.
Corn Stover-to-Ethanol Superstructure
Abbreviations—AFEX: ammonia fiber expansion, AHP: copper-catalyzed alkaline hydrogen peroxide, CB: combustor and boiler, COFER: co-fermentation, DA: dilute acid, EA: extractive ammonia, GVL: γ-valerolactone, HYD: hydrolysis, LV: lignin valorization, SEP: separation, SSCF: simultaneous saccharification and co-fermentation, SV: stillage valorization, TBG: turbogenerator, WWT: wastewater treatment.
Based on our literature review, we determine base values for the parameters describing the lignin depolymerization, monomer upgrading, and bioproduct separation process, defined collectively as the lignin valorization (LV) block. However, to keep the analysis general and given the scarcity of detailed techno-economic analysis (TEA) studies for lignin-based chemical production, we do not specify the target bioproducts and conversion and separation technologies used in the LV block. Rather, we establish targets in terms of the four key parameters, thereby providing insights into critical areas of improvement for viable lignin valorization. We do so for strategies considering both depolymerization (Table 1) and monomer upgrading (Table 2).
Results and Discussion
Base Case
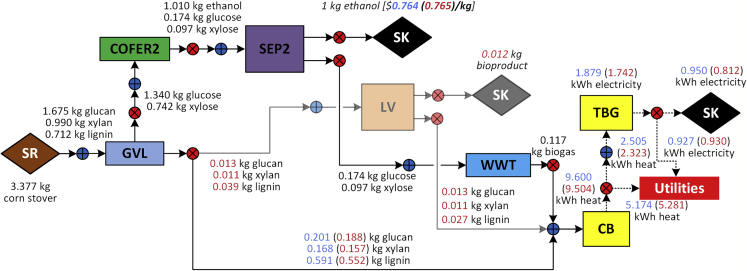
The material and energy balances for the optimal strategy under the base case parameters (see Tables S1–S3 for the base values of the parameters describing each block) are shown in Figure 2. Note that this strategy is the same as the base case strategy () reported by Ng et al. (2019), in which lignin valorization (LV block) is not selected. includes GVL, COFER2, SEP2, WWT, CB, and TBG1 blocks and has a minimum cost of $0.76/kg ethanol, which is equivalent to a minimum ethanol selling price (MESP) of $3.44/GGE. If we enforce the selection of lignin valorization by fixing the binary variable for the LV block (), the lignin stream from the GVL block will be split, and a fraction of it will be sent to LV satisfying the lower bound on production level. Thus, the optimal solution in this case, referred to as (see Figure 2), will depend on the selected lower capacity (e.g., ).
Figure 2.
Strategies (GVL-COFER2-SEP2-WWT-CB-TBG1) and (GVL-COFER2-SEP2-LV-WWT-CB-TBG1)
The faded arcs that are connected to the LV block are only applicable to the strategy. Black fonts before the SEP2 block are identical for both and strategies, whereas blue and red fonts after the SEP2 block represent the flows related to and strategies, respectively.
In the reminder of the paper, we study how changes in four key parameters for LV alter the optimal design, economics, and energy efficiency of a lignocellulosic biorefinery. In other words, we study how uncertainty in these parameters impact the optimal biorefinery configuration and its key performance metrics. Before we present the detailed results, we note that a number of other parameters are stochastic. However, although the impact of uncertainty in these parameters on the minimum ethanol cost can be significant (see Figure S4), the major insights of this study, in terms of technological targets, remain the same (see discussion in Transparent Methods). This is because these other parameters impact both the lignin-to-heat/power and lignin-to-bioproducts strategies. For example, an increase in feedstock price will lead to a more expensive lignin stream, but the fundamental trade-off we study (lignin-to-heat/power vs. lignin valorization) will not be noticeably impacted. Thus, although the minimum ethanol cost may change, the trends and the parameter values of the LV block at which strategy transitions occur will remain practically the same.
Analysis
To identify the major drivers for lignin valorization, we study the impact of the following parameters on the optimal biorefinery configuration and minimum ethanol cost: (1) conversion coefficient, (2) unit conversion cost, (3) bioproduct selling price, and (4) energy requirement. The first two depend on the efficiency of both conversion and separation technology employed within the valorization block, whereas the third one depends on the selected bioproduct. Additionally, the energy requirement depends on the energy input necessary for conversion (e.g., heat) and separation (e.g, heat and power). We note that, from a sustainability standpoint, a thermal-neutral biorefinery is always favorable, especially for biofuel, as opposed to biochemical, production (Humbird et al., 2011). Here, the term “thermal-neutral” refers to a biorefinery that is energetically self-sufficient through byproduct stream burning (e.g., unconverted lignin, biogas from anaerobic digestion, and biomass sludge from WWT) to generate steam and electricity and additional revenue through excess electricity sale (Humbird et al., 2011).
Conversion Coefficient and Bioproduct Selling Price
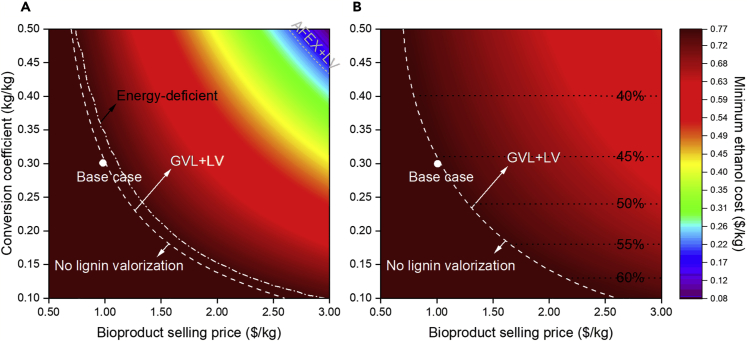
First, the sensitivity of the minimum ethanol cost is evaluated with respect to conversion coefficient and bioproduct selling price at a fixed unit conversion cost ($0.16/kg-lignin) and energy requirement (2.7 kWh/kg-lignin of heat and 0.05 kWh/kg-lignin of electricity) for the LV block. The results of this analysis are shown in Figure 3A. The minimum ethanol cost decreases with the increase of conversion coefficient and bioproduct selling price if the LV block is selected. In contrast, the minimum ethanol cost remains the same for the strategy at the bottom left region (region on the left of the white dashed line), which is selected when the conversion coefficient and bioproduct selling price are low. Figure 3 can also be used to predict the economic feasibility of a lignin valorization technology. For example, if a technology is developed to further convert intermediate bioproducts (e.g., monomers) to a high-value chemical via monomer upgrading (e.g., see strategies listed in Table 2), the selling price of the chemical should be at least $1.3/kg if 80% yield is achieved for this additional step (0.24 overall conversion coefficient).
Figure 3.
Minimum Ethanol Cost as a Function of Conversion Coefficient and Bioproduct Selling Price for the LV Block
(A) optimal solutions, based on economic metric, and (B) solutions that achieve a thermal-neutral biorefinery. White circle points indicate base case parameters; white dashed lines represent configuration transitions; gray dashed line represents pretreatment technology transition; white dash-dotted line represents thermal-neutral transition; black dotted lines represent lignin utilization percentage in the LV block.
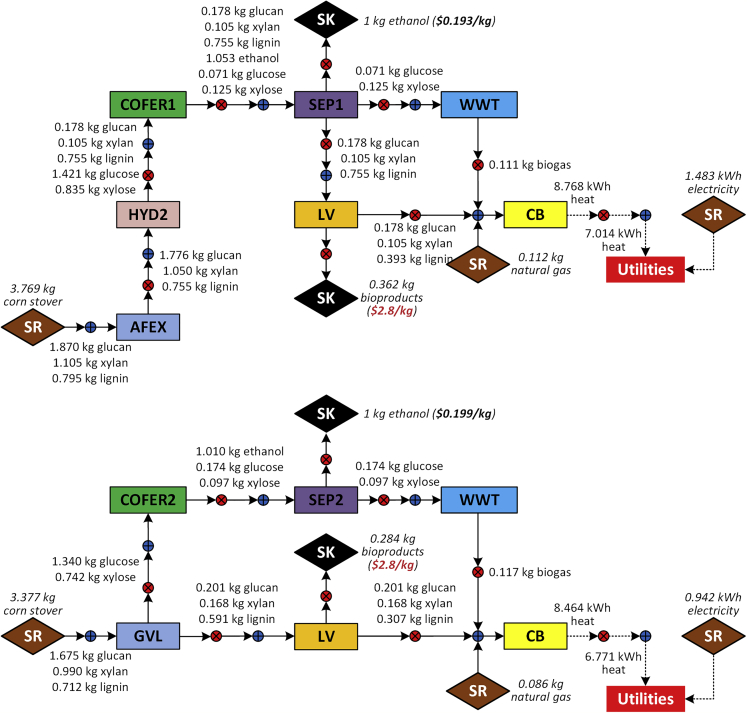
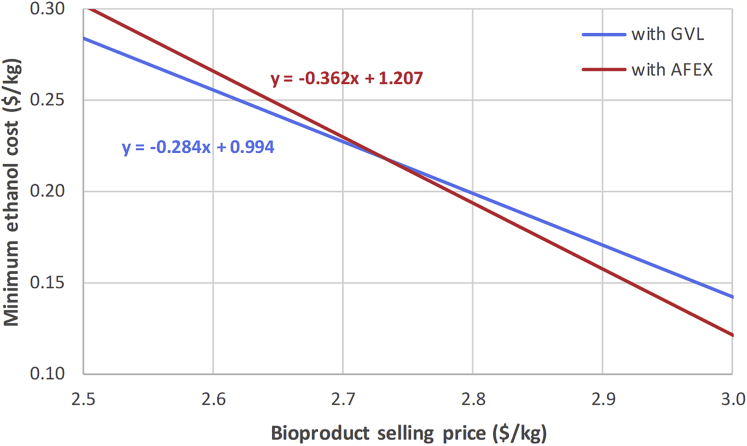
We also observe that the optimal pretreatment technology switches from GVL to AFEX at the top right region, above the gray dashed line, if both conversion coefficient (>0.43 kg-bioproduct/kg-lignin) and bioproduct selling price (>$2.6/kg) are high. Figure 4 shows the optimal strategies with the GVL block and AFEX block, respectively, based on the same conversion coefficient (0.48 kg-bioproduct/kg-lignin) and bioproduct selling price ($2.8/kg). The optimal strategy with the AFEX block has a higher product yield but also higher energy requirement than the one with the GVL block. To illustrate the trade off, we plot the minimum ethanol cost as a function of bioproduct price with fixed conversion coefficient for the two strategies (see Figure 5). Although the minimum ethanol cost of both strategies decreases with the bioproduct selling price, a higher product yield (0.362 kg-bioproduct/kg-ethanol vs 0.284 kg-bioproduct/kg-ethanol) leads to a faster reduction and it offsets the exceeded production cost when the bioproduct price goes beyond $2.73/kg.
Figure 4.
Optimal Configurations with the GVL Block and AFEX Block Using Same Conversion Coefficient (0.48 kg-Bioproduct/kg-Lignin) and Bioproduct Selling Price ($2.8/kg)
Figure 5.
Minimum Ethanol Cost as a Function of Bioproduct Price with Fixed Conversion Coefficient
The optimal strategies with lignin valorization tend to be energetically deficient because the generated energy from the remaining lignin is not sufficient to meet biorefinery demand (see region above and to the right of the white dashed dot line in Figure 3A). When LV is selected, natural gas and/or electricity is purchased because revenue from bioproducts sales is higher than the cost of purchased energy. Thus, we next consider strategies subject to the constraint that neither natural gas nor electricity is purchased. The strategy with the AFEX block is no longer selected (see Figure 3B) because it requires more energy than the one with the GVL block. Unlike the strategies leading to the results in Figure 3A, the optimal strategies with the LV block do not fully valorize lignin. Instead, a fraction of lignin is used for heat and power generation; the split ratio is determined by the conversion coefficient of the LV block. For example, 50% of lignin is used to produce bioproduct if the conversion coefficient is 0.24 and bioproduct selling price is greater than $1.2/kg. When the conversion coefficient increases, the utilization of lignin in the LV block decreases. Overall, when energy-related constraints are added, the optimal strategies with the LV block have higher minimum ethanol cost than those in Figure 3A. Because a thermal-neutral biorefinery is preferred, the following analysis focuses on the strategies with no externally purchased natural gas and/or electricity.
Production Cost and Bioproduct Selling Price
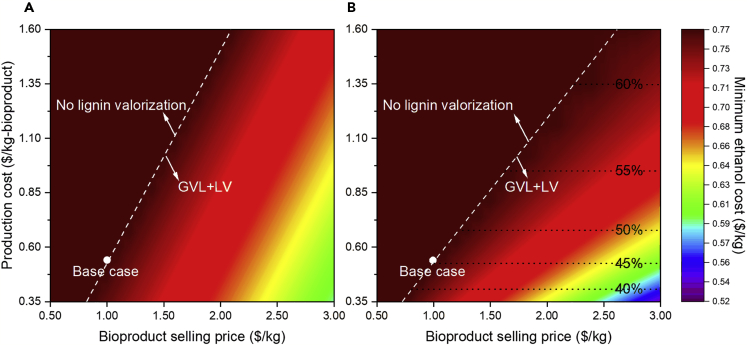
Here, for the LV block, we define the production cost ($/kg-bioproduct) as the quotient of the unit conversion cost ($/kg-lignin) by the conversion coefficient (kg-bioproduct/kg-lignin). This allows us to study the minimum ethanol cost as a function of the bioproduct selling price and production cost at a fixed conversion coefficient ($0.3 kg-bioproduct/kg-lignin) by changing its unit conversion cost. Clearly, higher bioproduct selling price and lower production cost lead to lower minimum ethanol cost. Because the conversion coefficient is fixed, the fraction of valorized lignin is 45%, whereas the remaining lignin is sent for heat and power generation. Similar to Figure 3B, the GVL block is preferred for pretreatment along with the LV block when the biorefinery is thermal-neutral.
Alternatively, we can plot the minimum ethanol cost in terms of the bioproduct selling price and production cost, considering a unit conversion cost equal to $0.162/kg-lignin but changing the conversion coefficient (see Figure 6B). Parameter combinations below and to the right of the white dashed line lead to lower minimum ethanol cost than those in Figure 6A. This indicates that conversion coefficient improvements are preferred over unit conversion cost reductions for the same production cost because an increase in bioproduct production leads to more revenue. Also, we see that the lignin utilization ratio is correlated with the conversion coefficient if the LV block is selected. For example, in Figure 6B, 50% lignin utilization corresponds to a production cost of $0.675/kg-bioproduct, which is equivalent to 0.24 kg-bioproduct/kg-lignin conversion coefficient when the unit conversion cost is fixed at $0.162/kg-lignin. This is consistent with the correlation between conversion coefficient and lignin utilization in Figure 6B.
Figure 6.
Minimum Ethanol Cost as a Function of Production Cost and Bioproduct Selling Price for the LV Block
Cost calculated by changing (A) unit conversion cost and fixing conversion coefficient at 0.3 kg-bioproduct/kg-lignin and (B) conversion coefficient and fixing unit conversion cost at $0.162/kg-lignin. White circle points indicate the base case parameters; white dash lines represent economic feasibility transitions; black dot lines represent lignin utilization percentage in the LV block.
Profit and Energy Requirement
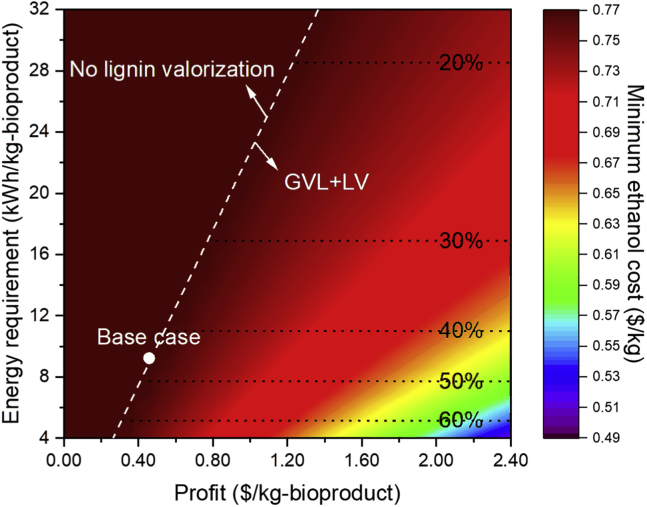
To understand the trade-offs between the economic and energy-related drivers, we combine all parameters considered in the previous sections in one parameter (called “profit”) and study its impact, along with the impact of energy requirement (kWh/kg-bioproduct), on the minimum ethanol cost. The profit ($/kg-bioproduct) for the LV block is calculated by
For illustration, we change the profit by varying the bioproduct selling price from $0.5–3.0/kg while keeping the other parameters at their base case values. The results are shown in Figure 7. No lignin valorization is selected when the combination of profit and energy requirement is on the left of the white dashed line. Furthermore, since the conversion coefficient is fixed, the lignin utilization percentage of the LV block is now correlated with its energy requirement. A higher energy requirement leads to a lower ratio of lignin valorized to bioproduct because more lignin would be required to fulfill the energy demand for a thermal-neutral biorefinery.
Figure 7.
Minimum Ethanol Cost as a Function of Profit and Energy Requirement in the LV Block
White circle points indicate the base case parameters; white dash line represents economic feasibility transitions; black dot lines represent lignin utilization percentage in the LV block.
Conclusion
In this paper, we studied biorefinery strategies for the conversion of biomass to ethanol coupled with lignin valorization subsystems. Based on process synthesis through superstructure optimization, thousands of optimizations were performed to evaluate the impact of various parameters related to lignin valorization on the optimal biorefinery configuration and minimum ethanol cost. Interestingly, we showed that different pretreatment technologies may be selected under different constraints. Our analysis provided baseline results and suggested what advances can make lignin valorization economically attractive. We hope that our results coupled with the identification of appropriate lignin-derived products and development of economical separation technologies for bioproduct recovery will help accelerate the development of lignin valorization technologies.
Limitations of the Study
Our goal is to identify general insights, and thus detailed analysis for specific lignin valorization products or strategies was not performed. However, detailed techno-economic analysis, including an assessment of the impact of uncertainty in key parameters (describing blocks other than LV), would become necessary when specific compounds and a detailed production process, including viable separation and recovery blocks, are identified.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This material is based upon work supported by the Great Lakes Bioenergy Research Center, U.S. Department of Energy, Office of Science, and Office of Biological and Environmental Research under Award Number DE-SC0018409.
Author Contributions
Conceptualization, C.T.M., K.H., and P.F.; Methodology, K.H., C.T.M., and P.F.; Software, K.H.; Formal Analysis, K.H.; Investigation, K.H., P.F., and C.T.M.; Resources, K.H., P.F., and C.T.M.; Writing—Original Draft, K.H. and P.F.; Writing—Review & Editing, K.H., P.F., and C.T.M.; Visualization, K.H. and C.T.M.; Supervision, C.T.M.; Funding Acquisition, C.T.M.
Declaration of Interests
The authors declare no competing interests.
Published: January 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.100751.
Supplemental Information
References
- Abdelaziz O.Y., Brink D.P., Prothmann J., Ravi K., Sun M., García-Hidalgo J., Sandahl M., Hulteberg C.P., Turner C., Lidén G., Gorwa-Grauslund M.F. Biological valorization of low molecular weight lignin. Biotechnol. Adv. 2016;34:1318–1346. doi: 10.1016/j.biotechadv.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Beckham G.T., Johnson C.W., Karp E.M., Salvachúa D., Vardon D.R. Opportunities and challenges in biological lignin valorization. Curr. Opin. Biotechnol. 2016;42:40–53. doi: 10.1016/j.copbio.2016.02.030. [DOI] [PubMed] [Google Scholar]
- Behling R., Valange S., Chatel G. Heterogeneous catalytic oxidation for lignin valorization into valuable chemicals: what results? What limitations? What trends? Green Chem. 2016;18:1839–1854. [Google Scholar]
- Boerjan W., Ralph J., Baucher M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003;54:519–546. doi: 10.1146/annurev.arplant.54.031902.134938. [DOI] [PubMed] [Google Scholar]
- Bugg T.D., Rahmanpour R. Enzymatic conversion of lignin into renewable chemicals. Curr. Opin. Chem. Biol. 2015;29:10–17. doi: 10.1016/j.cbpa.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Chakar F.S., Ragauskas A.J. Review of current and future softwood kraft lignin process chemistry. Ind. Crops Prod. 2004;20:131–141. [Google Scholar]
- Chandel A.K., Antunes F.A.F., Terán-Hilares R., Cota J., Ellilä S., Silveira M.H.L., dos Santos J.C., da Silva S.S. Bioconversion of hemicellulose into ethanol and value-added products. In: Chandel A.K., Silveira M.H.L., editors. Advances in Sugarcane Biorefinery. Elsevier; 2018. pp. 97–134. [Google Scholar]
- Da Costa Sousa L., Jin M., Chundawat S.P.S., Bokade V., Tang X., Azarpira A., Lu F., Avci U., Humpula J., Uppugundla N. Next-generation ammonia pretreatment enhances cellulosic biofuel production. Energy Environ. Sci. 2016;9:1215–1223. [Google Scholar]
- Gall D.L., Ralph J., Donohue T.J., Noguera D.R. Biochemical transformation of lignin for deriving valued commodities from lignocellulose. Curr. Opin. Biotechnol. 2017 doi: 10.1016/j.copbio.2017.02.015. [DOI] [PubMed] [Google Scholar]
- Gibson L.J. The hierarchical structure and mechanics of plant materials. J. R. Soc. Interface. 2012;9:2749–2766. doi: 10.1098/rsif.2012.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humbird D., Davis R., Tao L., Kinchin C., Hsu D., Aden A., Schoen P., Lukas J., Olthof B., Worley M. National Renewable Energy Laboratory (NREL); 2011. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover. [Google Scholar]
- Langan P., Gnanakaran S., Rector K.D., Pawley N., Fox D.T., Cho D.W., Hammel K.E. Exploring new strategies for cellulosic biofuels production. Energy Environ. Sci. 2011;4:3820. [Google Scholar]
- Langholtz M.H., Stokes B.J., Eaton L.M. U.S. Department of Energy; 2016. 2016 Billion-Ton Report: Advancing Domestic Resources for a Thriving Bioeconomy. [Google Scholar]
- Laskar D.D., Yang B., Wang H., Lee J. Pathways for biomass-derived lignin to hydrocarbon fuels. Biofuels, Bioprod. Biorefining. 2013;7:602–626. [Google Scholar]
- Li C., Zhao X., Wang A., Huber G.W., Zhang T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015;115:11559–11624. doi: 10.1021/acs.chemrev.5b00155. [DOI] [PubMed] [Google Scholar]
- Li Y., Shuai L., Kim H., Motagamwala A.H., Mobley J.K., Yue F., Tobimatsu Y., Havkin-Frenkel D., Chen F., Dixon R.A. An ideal lignin facilitates full biomass utilization. Sci. Adv. 2018;4 doi: 10.1126/sciadv.aau2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linger J.G., Vardon D.R., Guarnieri M.T., Karp E.M., Hunsinger G.B., Franden M.A., Johnson C.W., Chupka G., Strathmann T.J., Pienkos P.T., Beckham G.T. Lignin valorization through integrated biological funneling and chemical catalysis. Proc. Natl. Acad. Sci. U S A. 2014;111:12013–12018. doi: 10.1073/pnas.1410657111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R., Xu Y., Zhang X. Catalytic oxidation of biorefinery lignin to value-added chemicals to support sustainable biofuel production. ChemSusChem. 2015;8:24–51. doi: 10.1002/cssc.201402503. [DOI] [PubMed] [Google Scholar]
- Masai E., Katayama Y., Fukuda M. Genetic and biochemical investigations on bacterial catabolic pathways for lignin-derived aromatic compounds. Biosci. Biotechnol. Biochem. 2007;71:1–15. doi: 10.1271/bbb.60437. [DOI] [PubMed] [Google Scholar]
- Masai E., Kamimura N., Kasai D., Oguchi A., Ankai A., Fukui S., Takahashi M., Yashiro I., Sasaki H., Harada T. Complete genome sequence of sphingobium sp. strain SYK-6, a degrader of lignin-derived biaryls and monoaryls. J. Bacteriol. 2012;194:534–535. doi: 10.1128/JB.06254-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu W., Ben H., Ragauskas A., Deng Y. Lignin pyrolysis components and upgrading—technology review. BioEnergy Res. 2013;6:1183–1204. [Google Scholar]
- Mussatto S.I. Elsevier; 2016. Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery, Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery. [Google Scholar]
- Mycroft Z., Gomis M., Mines P., Law P., Bugg T.D.H. Biocatalytic conversion of lignin to aromatic dicarboxylic acids in Rhodococcus jostii RHA1 by re-routing aromatic degradation pathways. Green Chem. 2015;17:4974–4979. [Google Scholar]
- Ng R.T.L., Fasahati P., Huang K., Maravelias C.T. Utilizing stillage in the biorefinery: economic, technological and energetic analysis. Appl. Energy. 2019;241:491–503. [Google Scholar]
- Op de Beeck B., Dusselier M., Geboers J., Holsbeek J., Morré E., Oswald S., Giebeler L., Sels B.F. Direct catalytic conversion of cellulose to liquid straight-chain alkanes. Energy Environ. Sci. 2015;8:230–240. [Google Scholar]
- Peng F., Peng P., Xu F., Sun R.C. Fractional purification and bioconversion of hemicelluloses. Biotechnol. Adv. 2012 doi: 10.1016/j.biotechadv.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Perez J.M., Kontur W.S., Alherech M., Coplien J., Karlen S.D., Stahl S.S., Donohue T.J., Noguera D.R. Funneling aromatic products of chemically depolymerized lignin into 2-pyrone-4-6-dicarboxylic acid with: novosphingobium aromaticivorans. Green Chem. 2019;21:1340–1350. [Google Scholar]
- Rahimi A., Ulbrich A., Coon J.J., Stahl S.S. Formic-acid-induced depolymerization of oxidized lignin to aromatics. Nature. 2014;515:249–252. doi: 10.1038/nature13867. [DOI] [PubMed] [Google Scholar]
- Ralph J., Lapierre C., Boerjan W. Lignin structure and its engineering. Curr. Opin. Biotechnol. 2019 doi: 10.1016/j.copbio.2019.02.019. [DOI] [PubMed] [Google Scholar]
- Schutyser W., Renders T., Van den Bosch S., Koelewijn S.-F., Beckham G.T., Sels B.F. Chemicals from lignin: an interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018;47:852–908. doi: 10.1039/c7cs00566k. [DOI] [PubMed] [Google Scholar]
- Stolark J. Environmental and Energy Study Institute; 2017. If You Build it, They Will Come: Biofuels Industry Sees Renewable Chemicals as New Strategy [WWW Document] [Google Scholar]
- Sun Z., Fridrich B., de Santi A., Elangovan S., Barta K. Bright side of lignin depolymerization: toward new platform chemicals. Chem. Rev. 2018;118:614–678. doi: 10.1021/acs.chemrev.7b00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Energy Information Administration . Annual Energy Review 2009; 2018. History of Energy Consumption in the United States, 1775–2009 [WWW Document] [Google Scholar]
- UNGA . A New Era in Global Health. Springer Publishing Company; 2015. Transforming Our World: The 2030 Agenda for Sustainable Development. [Google Scholar]
- Upton B.M., Kasko A.M. Strategies for the conversion of lignin to high-value polymeric materials: review and perspective. Chem. Rev. 2016;116:2275–2306. doi: 10.1021/acs.chemrev.5b00345. [DOI] [PubMed] [Google Scholar]
- Vanholme R., Demedts B., Morreel K., Ralph J., Boerjan W. Lignin biosynthesis and structure. Plant Physiol. 2010;153:895–905. doi: 10.1104/pp.110.155119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardon D.R., Franden M.A., Johnson C.W., Karp E.M., Guarnieri M.T., Linger J.G., Salm M.J., Strathmann T.J., Beckham G.T. Adipic acid production from lignin. Energy Environ. Sci. 2015;8:617–628. [Google Scholar]
- Varman A.M., He L., Follenfant R., Wu W., Wemmer S., Wrobel S.A., Tang Y.J., Singh S. Decoding how a soil bacterium extracts building blocks and metabolic energy from ligninolysis provides road map for lignin valorization. Proc. Natl. Acad. Sci. U S A. 2016;113:E5802–E5811. doi: 10.1073/pnas.1606043113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiu S., Shahbazi A. Bio-oil production and upgrading research: a review. Renew. Sustain. Energy Rev. 2012;16:4406–4414. [Google Scholar]
- Yan N., Zhao C., Dyson P.J., Wang C., Liu L., Kou Y. Selective degradation of wood lignin over noble-metal catalysts in a two-step process. ChemSusChem. 2008;1:626–629. doi: 10.1002/cssc.200800080. [DOI] [PubMed] [Google Scholar]
- Zabed H., Sahu J.N., Boyce A.N., Faruq G. Fuel ethanol production from lignocellulosic biomass: an overview on feedstocks and technological approaches. Renew. Sustain. Energy Rev. 2016;66:751–774. [Google Scholar]
- Zakzeski J., Bruijnincx P.C.A., Jongerius A.L., Weckhuysen B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010;110:3552–3599. doi: 10.1021/cr900354u. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Song J., Han B. Catalytic transformation of lignocellulose into chemicals and fuel products in ionic liquids. Chem. Rev. 2017;117:6834–6880. doi: 10.1021/acs.chemrev.6b00457. [DOI] [PubMed] [Google Scholar]
- Zhao C., Xie S., Pu Y., Zhang R., Huang F., Ragauskas A.J., Yuan J.S. Synergistic enzymatic and microbial lignin conversion. Green Chem. 2016;18:1306–1312. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.