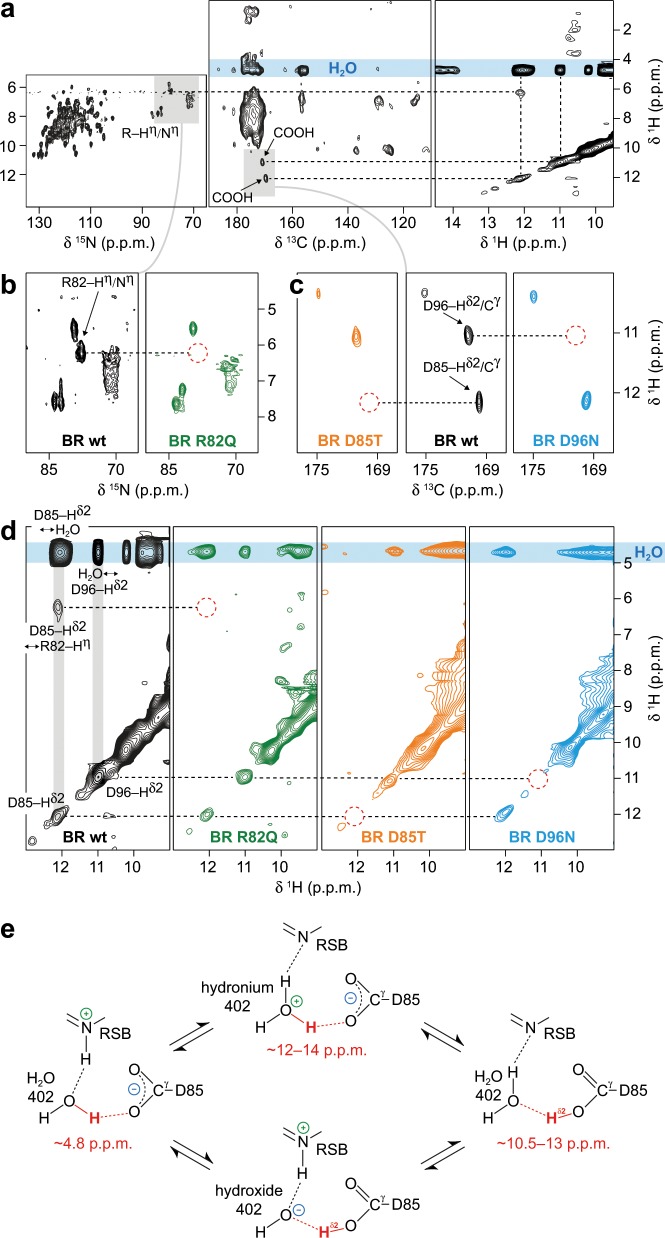
Fig. 2. Protonation states and chemical exchange of protons at R82, D96, D85, and water molecules in bacteriorhodopsin (BR) probed by proton-detected MAS NMR at room temperature.
a 15N–1H (left), 13C–1H (middle), and 1H–1H exchange (right) spectra of BR wildtype. b 15N–1H correlations of BR wildtype (wt, black spectrum) and the mutant R82Q (green spectrum). c 13C–1H correlations of BR wildtype (wt, black spectrum) and the D85T and D96N mutants (orange and blue spectra, respectively). The mutants are used to assign the signals of D96–Hδ2 (11.0 ppm), D85–Hδ2 (12.1 ppm) and R82–Hη (6.2 ppm). Red dashed circles indicate missing signals resulting from mutations that allow the assignment. d 1H–1H exchange spectra of BR wildtype (wt, black spectrum), R82Q (green spectrum), D85T (orange spectrum) and D96N (blue spectrum). e At H2O 402, both hydronium and hydroxide ions are possible, affecting the proton localization through exchange within the hydrogen bonds to the retinal Schiff base (RSB) and D85. The observed chemical shifts for the proton highlighted in red agree with tautomeric structures involving H2O 402, a hydronium 402 and a carboxyl group proton at D85.