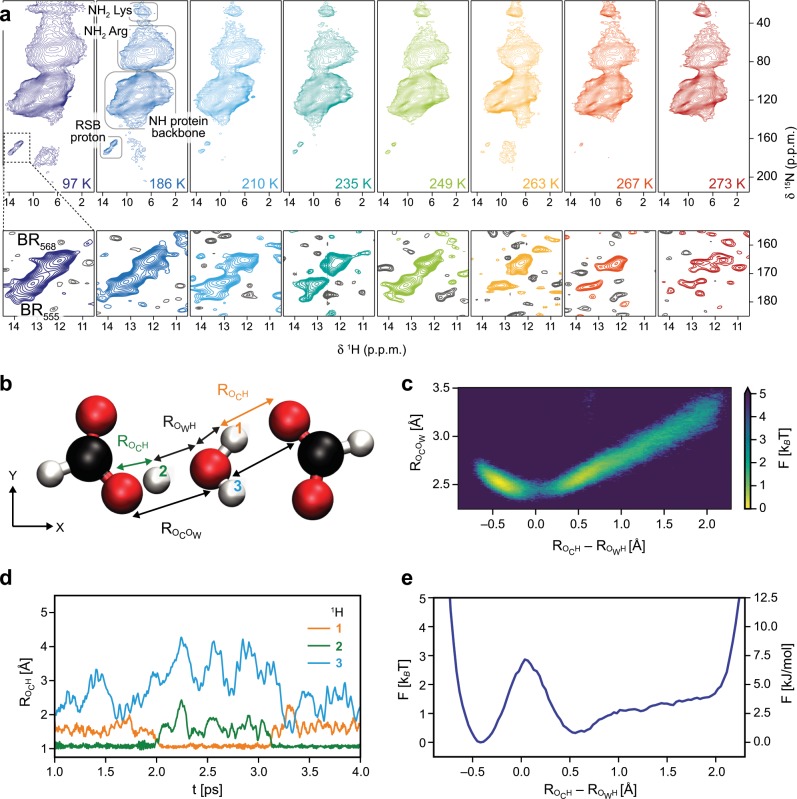
Fig. 3. Observation of retinal Schiff base (RSB) proton dynamics by MAS NMR and characterization of proton exchange by ab initio molecular dynamics simulations in a model system.
a Proton-detected, two-dimensional 15N–1H correlation spectra of purple membrane in bacteriorhodopsin (BR) dark-state recorded at temperatures ranging from 97 K (dark blue spectrum) to 273 K (red spectrum). The spectral region of the RSB proton signals for BR568 and BR555 is plotted at the noise level (bottom panels, positive contours are color-coded according to the temperature and negative contours are shown in gray). b The proton distribution is analyzed by ab initio molecular dynamics simulations in a model system consisting of one H2O molecule, two carboxyl groups and one excess proton which are all subject to thermal positional fluctuations. The distance between carboxyl group and water oxygen atoms (Rocow) and the excess proton’s relative asymmetry with respect to the two oxygens (Roch–Rowh) are used as effective reaction coordinates. c The free energy landscape of the proton is shown as a function of the coordinates, as defined in b. d Trajectories of the distances, Roch, of the three central hydrogens labeled as in b to the respective closest carboxyl group oxygen. The proton exchange is well visible as a fast jump process: proton 1 resides near the closest carboxyl group oxygen for about 1 ps in the time interval from t = 2.0 ps to t = 3.1 ps. e The free energy of the protons projected onto the asymmetry coordinate (Roch–Rowh) indicates a low proton transfer barrier of about 3 kBT.