Fig. 1. The venemycin synthase viewed from several perspectives.
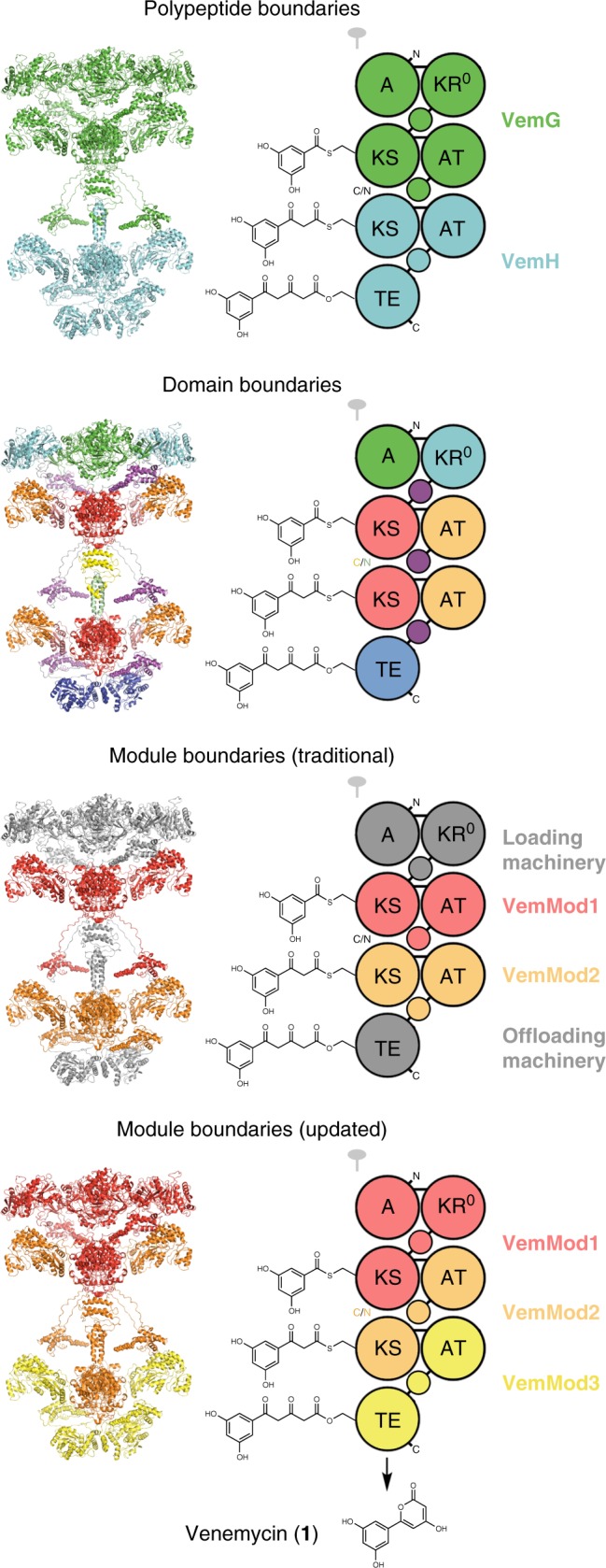
Its polypeptides, the 232 kDa VemG and the 140 kDa VemH, are threaded through known structures to create an all-atom model of this short polyketide assembly line. An adenylation (A) domain accepts a 3,5-dihydroxybenzoyl starter unit, and malonyl groups, transferred from malonyl-CoA to acyl carrier protein (ACP) domains (small circles) by acyltransferases (ATs), extend the growing chain within the active sites of ketosynthases (KSs). A thioesterase (TE) catalyzes cyclization to yield the aromatic product venemycin. C- and N-terminal docking domains are represented by C/N. An inactive ketoreductase (KR0) may play a structural role. The flanking subdomains (also known as KS/AT adapters in cis-AT PKSs), which are C-terminal to KS domains, appear pink in the model but are not depicted in the cartoon. Traditional module boundaries run from the N-terminal end of KS domains to the C-terminal end of ACP domains. Recently, module boundaries were redefined at the C-terminal end of KS domains to reflect the evolutionary co-migration of and collaboration between assembly line domains.
