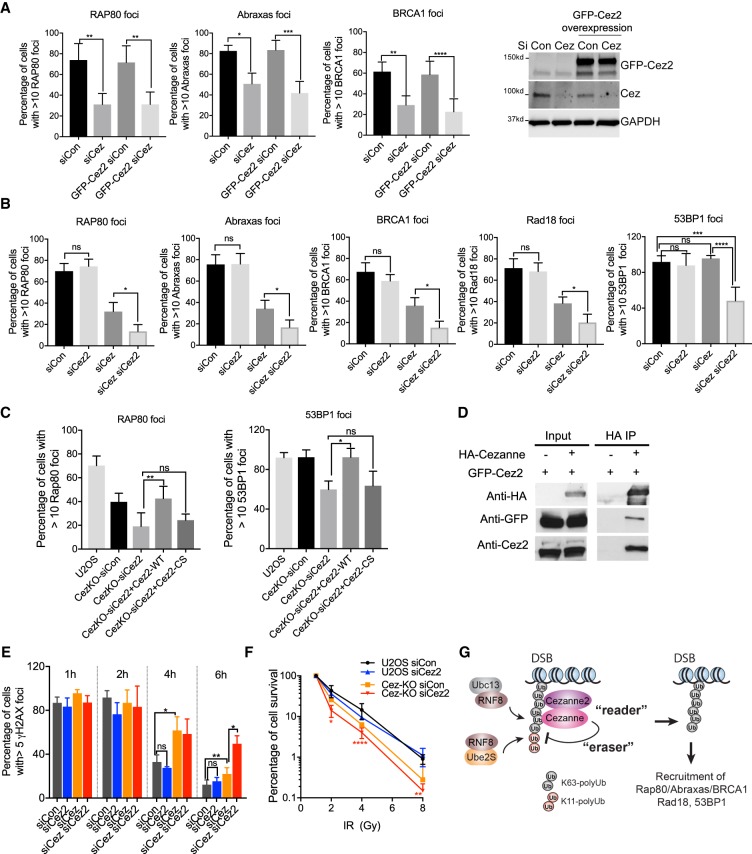
Figure 7.
Cezanne and Cezanne2 play critical roles in recruitment of Rap80/Abraxas/BRCA1, Rad18 and 53BP1, cellular resistance to IR, and DNA damage repair. (A) Overexpression of GFP-Cezanne2 does not rescue Cezanne-deficiency in recruitment of Rap80/Abraxas/BRCA1. U2OS or U2OS cells overexpressing GFP-Cezanne2 were transfected with control or Cezanne siRNA, treated with 10 Gy IR, and incubated for 2 h before fixation and staining. Nonparametric Kruskal–Wallis ANOVA was used for statistical analysis. (B) Cezanne and Cezanne2 promote recruitment of Rap80/Abraxas/BRCA1, Rad18, and 53BP1. Cells were treated with 10 Gy IR, followed by 2 h incubation. Nonparametric Kruskal–Wallis ANOVA was used for statistical analysis. (C) Cezanne2 DUB activity is required for its role in promoting recruitment of Rap80 and 53BP1. Cezanne KO cells were transfected with Cezanne2 siRNA and complemented with expression of siRNA-resistant Cezanne2 WT or the DUB mutant (Cez2-CS) construct. Forty-eight hours after transfection, cells were treated with 10 Gy IR and incubated for 2 h. Nonparametric Kruskal–Wallis ANOVA was used for statistical analysis. (D) Cezanne interacts with Cezanne2. 293T cells were cotransfected with GFP-Cezanne2 and vector or HA-Cezanne expression plasmids. Total lysates were used for immunoprecipitation. (E) Cezanne and Cezanne2 are required for efficient DNA repair. Cells were treated with 2 Gy IR, fixed, and stained with γ-H2AX antibody at indicated times. Nonparametric Kruskal–Wallis ANOVA was used for statistics analysis. (F) Colony survival assay for cells treated with indicated doses of IR. Two-way ANOVA statistical analysis was performed to compare Cezanne-deficient (Cez-KO siCon, orange) with Cezanne- and Cezanne2-double deficient (Cez-KO siCez2, red) cells. (G) A proposed model showing that Cezanne and Cezanne2 promote recruitment of Rap80/Abraxas/BRCA1, Rad18, and 53BP1 through binding to K63-linked ubiquitin (“reader”) antagonizing K11-linked ubiquitin conjugation (“eraser”) at DNA damage sites.