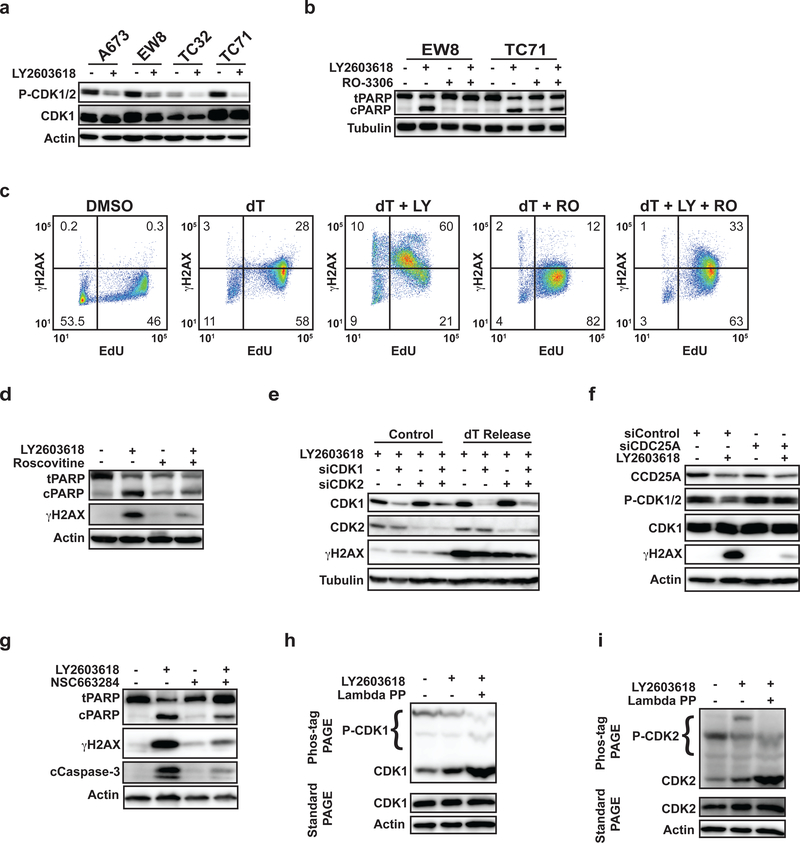
Figure 2.
Inhibition of the ATR-CHK1 pathway in Ewing sarcoma cells in S phase activates CDK1/2. (A) Multiple Ewing sarcoma cell lines were released from a thymidine double block in the presence of LY2603618 (1 μM) or DMSO. Cellular lysates were harvested 4 h after release from the block and probed for CDK1/2 phosphorylation by immunoblotting. (B) EW8 and TC71 cells were released from a thymidine double block in the presence of LY2603618 (1 μM), RO-3306 (10 μM), or the combination of LY2603618 and RO-3306. Cellular lysates were collected for immunoblotting 4 h after release from the block. (C) EW8 cells were released from a thymidine double block in the presence of LY2603618 (1 μM), RO-3306 (10 μM), or the combination of LY2603618 and RO-3306 for 4 h. Cells were then labeled with EdU and fixed for flow cytometry for γH2AX. Results are representative of two independent experiments. (D) EW8 cells were released from a thymidine double block in the presence of LY2603618 (1 μM), Roscovitine (10 μM), or the combination of LY2603618 and Roscovitine. Cellular lysates were collected for immunoblotting 4 h after release from the block. (E) EW8 cells were released from a thymidine double block 24 h after transfection with siRNA targeting CDK1, CDK2, or CDK1 and CDK2. Cellular lysates were collected 4 h after the cells were released from the block in the presence of LY2603618 and probed for CDK1, CDK2, and γH2AX. (F) EW8 cells were released from a thymidine double block 24 h after transfection with siRNA targeting CDC25A. Cellular lysates were collected 4 h after the cells were released from the block in the presence of LY2603618 and probed for CDC25A, CDK1, P-CDK1/2, and gH2AX. (G) EW8 cells were released from a thymidine double block in the presence of LY2603618 (1 μM), NSC663284 (10 μM), or the combination of LY2603618 and NSC663284. Cellular lysates were collected for immunoblotting 4 h after release from the block. (H, I) EW8 cells were released from a thymidine double block in the presence of LY2603618 and then cellular lysates were collected 4 h after release. Electrophoresis was performed using phos-tag gels and the gels were probed with CDK1 (H) and CDK2 (I) antibodies. As a control, an aliquot of protein lysate was treated with lambda phosphatase. The same lysates were also evaluated using standard polyacrylamide gel electrophoresis, which does not separate the unphosphorylated and phosphorylated CDK1 and CDK2 proteins, to ensure that the levels of total CDK1 and CDK2 are not altered by drug treatment.