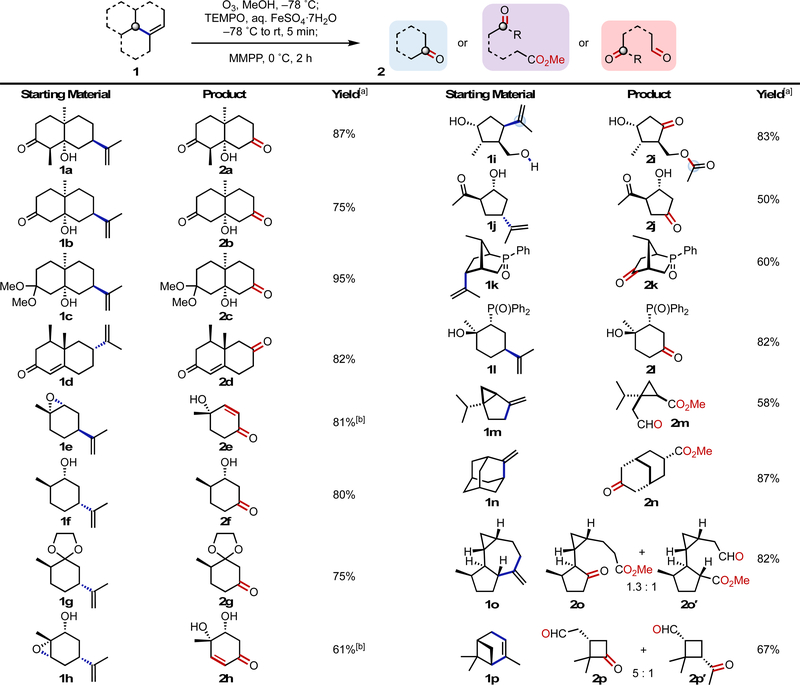
Scheme 3.
Examples of oxodealkenylation. Reaction conditions: alkene 1 (0.5–2.0 mmol, 1.0 equiv) in MeOH (0.025 M), TEMPO (1.5 equiv), aq. FeSO4·7H2O (5% wt/vol, 1.2 equiv), MMPP (2.5 equiv). See the SI for further experimental details. [a] Isolated yields after SiO2 chromatography. [b] The crude reaction mixture was treated with Et3N during workup.