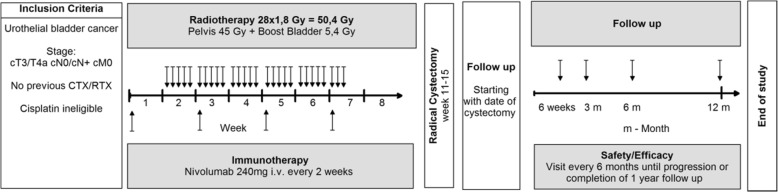
Fig. 1.
Schematic outline of the treatment plan. Patients with locally advanced bladder cancer, included in RACE IT study, will receive Nivolumab 240 mg i.v. every 2 weeks for 4 cycles preoperatively with concomitant radiation therapy of bladder and pelvic region (max. 50.4 Gy) Radical cystectomy with standardized bilateral pelvic lymphadenectomy will be performed between week 11–15. Follow up for secondary endpoints will be starting at the date of cystectomy for 1 year every 6 months