Abstract
Although accumulating evidence has indicated the intimate association between epithelial‐mesenchymal transition (EMT) and acquired resistance to chemotherapy for colorectal cancer (CRC), the underlying mechanisms remain elusive. Herein, we reported that Snail, a crucial EMT controller, was upregulated in CRC tissues. Colorectal cancer cells overexpressing Snail were found to be more resistant to 5‐fluorouracil (5‐Fu). Mechanistic studies reveal that Snail could increase the expression of ATP‐binding cassette subfamily B member 1 (ABCB1) rather than the other 23 chemoresistance‐related genes. Additionally, knockdown of ABCB1 significantly attenuated Snail‐induced 5‐Fu resistance in CRC cells. Oxaliplatin increased Snail and ABCB1 expression in CRC cells. Snail and ABCB1 were upregulated in 5‐Fu‐resistant HCT‐8 (HCT‐8/5‐Fu) cells and inhibition of Snail decreased ABCB1 in HCT‐8/5‐Fu cells. These results confirm the vital role played by ABCB1 in Snail‐induced chemoresistance. Further investigation into the relevant molecular mechanism revealed Snail‐mediated ABCB1 upregulation was independent of β‐catenin, STAT3, PXR, CAR and Foxo3a, which are commonly involved in modulating ABCB1 transcription. Instead, Snail upregulated ABCB1 transcription by directly binding to its promoter. Clinical analysis confirms that increased Snail expression correlated significantly with tumor size (P = .018), lymph node metastasis (P = .033), distant metastasis (P = .025), clinical stage grade (P = .024), and poor prognosis (P = .045) of CRC patients. Moreover, coexpression of Snail and ABCB1 was observed in CRC patients. Our study revealed that direct regulation of ABCB1 by Snail was critical for conferring chemoresistance in CRC cells. These findings unraveled the mechanisms underlying the association between EMT and chemoresistance, and provided potential targets for CRC clinical treatment.
Keywords: ABCB1, chemoresistance, colorectal cancer, EMT, Snail
Snail is upregulated in colorectal cancer (CRC) tissues and correlates significantly with the tumor size, lymph node metastasis, distant metastasis, clinical stage grade, and poor prognosis of CRC patients. Snail induces drug resistance in CRC by upregulation of ABCB1 expression. Snail regulates ABCB1 transcription by directly binding to its promoter.
1. INTRODUCTION
The epithelial‐mesenchymal transition (EMT) is an important phenotypic conversion that occurs at the invasive front of many metastatic cancers.1, 2, 3 Several transcription factors, including Snail, Slug, Twist, and ZEB1, trigger the EMT by restraining the expression of E‐cadherin.4, 5 Snail is overexpressed in various metastatic cancer cells and has been identified as a crucial regulator of the EMT.6, 7 Clinically, patients with metastatic tumors are more resistant to chemotherapy and have a poor therapeutic response. Emerging evidence suggests that the EMT phenotype is associated with the acquisition of chemoresistance in cancer cells. For instance, overexpression of Twist contributes to chemoresistance in breast cancer cells and colorectal cancer (CRC) cells.8, 9 It has been shown that ZEB1 can promote the resistance of pancreatic cancer cells to cisplatin, gemcitabine, and 5‐fluorouracil (5‐Fu).10 Moreover, the susceptibility of cancer cells with the mesenchymal phenotype to chemotherapeutic agents is markedly lower than that of cancer cells with the epithelial phenotype.11 We have previously reported that lung cancer cells resistant to cisplatin acquire an EMT phenotype and cancer stem cell‐like properties.12 Taken together, these studies indicate a correlation between the EMT and chemoresistance. However, the underlying mechanisms are still unclear.
Tumor metastasis and the development of chemoresistance are responsible for the mortality due to CRC, one of the leading causes of cancer‐driven death. Despite the efficacy of initial chemotherapy for most CRC patients, the response rate significantly decreases with the length of treatment. Hence, there is an urgent demand for elucidating the molecular mechanisms of tumor metastasis and chemoresistance, which is important for exploring innovative remedies.
The molecular mechanisms involved in the development of chemoresistance are highly complicated and include decreased intracellular accumulation of drugs, suppression of cell apoptosis, overexpression of DNA repair enzymes, and activation of DNA damage checkpoint responses.13, 14 Among these, ATP‐binding cassette (ABC) membrane transport protein‐mediated efflux of intracellular cytotoxic agents has been proven to be one of the most important factors regulating chemoresistance.15, 16 The first discovered ABC transporter, ABCB1, also known as multidrug resistance protein 1 (MDR1), is capable of exporting a variety of antitumor drugs out of cancer cells and thereby inducing multidrug resistance.17, 18 Importantly, mounting studies have indicated that ABCB1 overexpression culminates in chemotherapy failure in a wide variety of cancer cells, exemplified by CRC cells.19, 20
Our group has been continuously dedicated to research of the molecular mechanisms associated with chemoresistance in cancer therapy. We previously uncovered the role of Snail in controlling the EMT phenotype and cancer stem cell features in cisplatin‐resistant lung cancer cells.12 Considering the mortality of chemoresistant CRC, the present study has focused on an unexplored mechanism, namely, how Snail‐overexpressing CRC evades treatment with cytotoxic agents, in an attempt to better understand the association of the EMT with chemoresistance.
2. MATERIALS AND METHODS
2.1. Chemicals and reagents
The SimpleChIP Enzymatic Chromatin IP Kit and primary Abs against ABCB1, Foxo3a, and STAT3 were obtained from Cell Signaling Technology. Human Snail Ab was purchased from R&D Systems. Primary Abs against β‐catenin, pregnane X receptor (PXR), constitutive androstane receptor (CAR), and α‐tubulin were obtained from Santa Cruz Biotechnology. The PrimeScript RT Reagent Kit and SYBR Premix Ex Taq were purchased from Takara Bio. Smartpool siRNA against ABCB1 was obtained from RiboBio. Vectors (pGL3‐Basic, pcDNA3.1, and pRL‐TK) and a dual‐luciferase assay kit were purchased from Promega.
2.2. Cell culture
The cell lines HCT116, HT29, SW480, HCT‐8, and HEK293T were obtained from the Type Culture Collection of the Chinese Academy of Sciences. The human CRC‐resistant cell line HCT‐8/5‐Fu was established in our laboratory. Briefly, HCT‐8 cells were treated with a series of stepwise‐increased concentrations of 5‐Fu to develop a HCT‐8/5‐Fu‐resistant cell line. HCT116 and HT29 cells were maintained in DMEM/F12 culture medium supplemented with 10% FBS, SW480 and HEK293T cells were cultured in DMEM culture medium supplemented with 10% FBS, and HCT‐8 and HCT‐8/5‐Fu cells were maintained in RPMI‐1640 culture medium supplemented with 10% FBS under a humidified 5% CO2 atmosphere at 37°C in an incubator.
2.3. Quantitative real‐time PCR
After treatment for the indicated time, the total mRNA of cells was extracted with TRIzol reagent and 500 ng RNA was used for cDNA synthesis. Quantitative real‐time PCR using the SYBR Premix Ex Taq real‐time PCR system (Takara Bio) was applied to quantify the expression of target genes on an ABI 7500 system (Applied Biosystems). GAPDH was selected as the reference gene. The sequences of the primers used in the real‐time PCR experiments are shown in Table 1.
Table 1.
Primers used in real‐time PCR assay
| Gene | Forward primer 5′–3′ | Reverse primer 5′–3′ |
|---|---|---|
| Snail | GACCACTATGCCGCGCTCTT | TCGCTGTAGTTAGGCTTCCGATT |
| ABCB1 | TGCTCAGACAGGATGTGAGTTG | AATTACAGCAAGCCTGGAACC |
| ABCC1 | GCCAAGAAGGAGGAGACC | AGGAAGATGCTGAGGAAGG |
| ABCC2 | TGGTGGCAACCTGAGCATAGG | ACTCGTTTTGGATGGTCGTCTG |
| ABCC3 | CTTAAGACTTCCCCTCAACATGC | GGTCAAGTTCCTCTTGGCTC |
| ABCC4 | GGTTCCCCTTGGAATCATTT | AATCCTGGTGTGCATCAAACAG |
| ABCC5 | ACCCGTTGTTGCCATCTTAG | GCTTTGACCCAGGCATACAT |
| ABCC6 | GTGGTGTTTGCTGTCCACAC | ACGACACCAGGGTCAACTTC |
| ABCC10 | ATTGCCCATAGGCTCAACAC | AGCAGCCAGCACCTCTGTAT |
| ABCC11 | GGCTGAGCTACTGGTTGGAG | TGGTGAAAATCCCTGAGGAG |
| ABCC12 | GGTGTTCATGCTGGTGTTTGG | GCTCGTCCATATCCTTGGAA |
| ABCG2 | TATAGCTCAGATCATTGTCACAGTC | GTTGGTCGTCAGGAAGAAGAG |
| ERCC1 | CTCAAGGAGCTGGCTAAGATGT | CATAGGCCTTGTAGGTCTCCAG |
| ERCC2 | CTGGAGGTGACCAAACTCATCTA | CCTGCTTCTCATAGAAGTTGAGC |
| XRCC1 | CATCGTGCGTAAGGAGTGGGTG | AGTGGGCTTGGTTTTGGTCTGG |
| Mcl‐1 | CGCCAAGGACACAAAGCC | GTCTCGTGGTTGCGCTGC |
| BRCA‐1 | GCCAGAAAACACCACATCAC | CAGTGTCCGTTCACACACAA |
| BRCA‐2 | CTCTGCCCTTATCATCGCTTTTC | TTTTGCTGCTTCCTTTTCTTCCT |
| MSH‐2 | GCTTCTCCTGGCAATCTCTCTC | TACCCAACTCCAACCTGTCTCT |
| MSH‐6 | TCATAAATGCTGAAGAACGGA | ACAGAATTACTGGGCGACACA |
| TUBB3 | TCAGCAAGGTGCGTGAGGAGTAT | CGGAAGCAGATGTCGTAGAGCG |
| GST | ACCTCCGCTGCAAATACATC | CTCAAAAGGCTTCAGTTGCC |
| γGT1 | GCCTGGATTCTCCCAGAGAT | GGAGAGCACCTCTTCCTCAG |
| GADD45α | GGATGCCCTGGAGGAAGTGCT | GGCAGGATCCTTCCATTGAGATGAATGTG |
| GADD45β | CCACTTCACGCTCATCCAGTCCTT | CAGGCGTCCGTGTGAGGGTTC |
| E‐cadherin | TACACTGCCCAGGAGCCAGA | TGGCACCAGTGTCCGGATTA |
| β‐Catenin | GCGTTCTCCTCAGATGGTGTC | CCAGTAAGCCCTCACGATGAT |
| Foxo3a | GCGTGCCCTACTTCAAGGA | GACCCGCATGAATCGACTATG |
| STAT3 | ACATTCTGGGCACAAACACA | CAGTCACAATCAGGGAAGCA |
| PXR | CAAGCGGAAGAAAAGTGAAC | TGAAATGGGAGAAGGTAGTG |
| CAR | ACTTTCTGTCTCCAAACACA | GCAACTCCAAAAACTCTACC |
| GAPDH | GCACCGTCAAGGCTGAGAAC | TGGTGAAGACGCCAGTGGA |
2.4. Western blot analysis
After being washed with ice‐cold PBS, the cells were lysed and harvested. Equal amounts of protein samples were loaded on SDS‐polyacrylamide gels and then transferred onto PVDF membranes. After being blocked with 5% blocking buffer at room temperature for 2 hours, the membranes were probed with primary Abs at 4°C overnight and then incubated with secondary Abs for 1.5 hours at room temperature. The signals of specific immune complexes were detected using electrochemiluminescence reagent.
2.5. Dual‐luciferase reporter assay
HCT116 cells were transiently cotransfected with a pGL3‐Basic‐ABCB1, pRL‐TK, pcDNA3.1‐Snail, or empty pcDNA3.1 vector. pRL‐TK, which expresses Renilla luciferase, was cotransfected in each experiment as an internal control. After 48 hours, the cells were harvested and the activities of firefly luciferase and Renilla luciferase were detected by the dual‐luciferase reporter assay system. The promoter activities were calculated by normalization of the ratio between the activities of firefly luciferase and Renilla luciferase. The sequences of primers used in the mutagenesis assay were as follows: ABCB1‐mut1‐F, ATTTCTCTATCGATAGGTACCGGTATATCCAGTGCATTGTTG; ABCB1‐mut1‐r, AGCTTTATGGAAATTGTAAACTTTACTTTGCAATTATATCAGTATTTAATTTATAATG; ABCB1‐mut1‐f, TAATTGCAAAGTAAAGTTTACAATTTCCATAAAGCTAATTTATCTTTATATTTTC; and ABCB1‐mut1‐R, ACTTAGATCGCAGATCTCGAGGAGTATTTGTACCTTACC. The sequence of the E‐box motif is “CAAATG” (−577 to −571), and the sequence of mutated E‐box motif is “GTTTAC”.
2.6. Cell viability assay
Cell viability was measured by using the CCK‐8 agent. Briefly, cells were seeded in 96‐well plates at a density of 1 × 104 cells/well and treated with different concentrations of 5‐Fu for 48 hours. The 10 μL CCK‐8 agent was added to each well and the plates were incubated for 4 hours at 37°C. The absorbance was detected at 570 nm using a microplate reader. All experiments were carried out in triplicate.
2.7. Chromatin immunoprecipitation assay
HCT116 cells transfected with pcDNA‐Snail were harvested after 48 hours and cross‐linked with 1% formaldehyde for 15 min at room temperature. Chromatin fragments ranging from 200 to 800 bp were obtained by sonication. The protein‐DNA complex was precipitated by anti‐Snail Ab or anti‐IgG Ab at 4°C overnight. Then the Ab‐protein‐DNA complex was collected by protein beads. After being eluted from the beads, the Ab‐protein‐DNA complex was reverse cross‐linked by incubation at 65°C with 200 mmol/L NaCl. The amount of immunoprecipitated DNA was detected by semiquantitative PCR. The primer sequences used in the PCR experiments were as follows: ABCB1 sense, 5′‐TCTGTGACAGCTCAGRCATTTAC‐3′; ABCB1 antisense, 5′‐AAAAAGTTGTGTTCCAAAGAAATGT‐3′.
2.8. Immunohistochemistry
Colorectal cancer tumor tissues were collected from 46 patients at Anhui Provincial Hospital. The use of human tumor tissue and clinical data was approved by the Affiliated Anhui Provincial Hospital of Anhui Medical University’s Ethics Committees. All patients gave their written informed consent. Sections cut from the tumor tissues were subjected to deparaffinization/rehydration and antigen retrieval by boiling in 0.01 mol/L sodium citrate buffer for 30 minutes. The sections were blocked with 10% goat serum and then incubated with primary Abs against Snail and ABCB1 at a 1:200 dilution at 4°C overnight in a humidified chamber. After being washed 3 times with PBS, slides were incubated with HRP‐conjugated Ab; DAB was used as substrate and Mayer’s hematoxylin was applied as a counterstain. Throughout the above analyses, controls were prepared by omitting the primary Abs.
The expression levels of Snail and ABCB1 were independently evaluated by 2 pathologists. The tumor cells with brown cytoplasm, nucleus, or membrane were considered positive. These cells were scored and then classified into the following 4 classes: none (0), weak brown (1+), moderate brown (2+), and strong brown (3+). The percentage of the stained tumor cells was divided into the following 5 classes: 0, negative cells; 1, 1%–25%; 2, 25%–50%; 3, 50%–75%; and 4, >75%. The multiplication (staining index) of the intensity and percentage scores was used to determine the result.
2.9. Database search
Data about the expression of Snail in CRC and normal tissues were obtained from the Gene Expression Omnibus (GEO) database (GSE32323). Data about the expression of ABCB1 in CRC and normal tissues and the correlation of ABCB1 and prognosis of CRC patients were obtained from GSE24551. Data about the correlation of Snail and ABCB1 expression were obtained from GSE75315. Data about Snail gene expression in CRC and normal tissues, Snail expression with clinicopathological factors, and prognosis were obtained from The Cancer Genome Atlas (TCGA) online database (http://cancergenome.nih.gov). Samples with incomplete clinical information were excluded. Survival analyses with gene expression were undertaken in R using the survival package RStudio, Version 1.1.442 (RStudio Inc.). The gene expression association to survival was evaluated by fitting a Cox proportional hazards regression model. The explanatory variable of the survival model fitted for each gene was either the continuous expression values or the discretized expression value (eg, below vs over the median expression). Kaplan‐Meier analysis was carried out to estimate the survival curves of the different subgroups and the log‐rank test (Mantel‐Cox) was used to compare the curve.
2.10. Statistical analysis
Results are expressed as the mean ± SD of 3 independent experiments unless otherwise specified. Differences between any 2 groups were analyzed by a 2‐tailed unpaired Student’s t test. One‐way ANOVA was used to assess the difference in means among groups. Pearson’s χ2 test was used to measure the expression difference of Snail in CRC tumor samples and adjacent normal tissues. The Wilcoxon test and Kruskal‐Wallis test were used to assess the correlations between expression levels of Snail and clinicopathological characteristics of CRC. The Wilcoxon test was used to assess the difference between 2 groups and the Kruskal‐Wallis test was used to assess the difference in means among groups. Survival curves were generated using the Kaplan‐Meier method. Pearson’s correlation coefficient test was used to assess the correlation between 2 continuous variables. These analyses were carried out using GraphPad Prism version 5.0 software (GraphPad Software). P < .05 was considered statistically significant.
3. RESULTS
3.1. Snail is upregulated in CRC and predicts poor survival of patients
We first investigated the expression of Snail in 46 pairs of CRC tumor samples and its matched adjacent normal tissues. The results of immunohistochemistry showed that Snail expression was significantly increased in CRC tumor samples compared to adjacent normal tissues (Figures 1A and S1). We analyzed TCGA and GEO patient databases and found that Snail mRNA expression was higher in CRC tissues than in normal tissues (Figure 1B,C). The GEO data showed that expression of Snail in CRC was significantly higher than their matched adjacent normal tissues (Figure 1D). Furthermore, TGGA data showed the association between Snail expression and clinicopathologic variables of CRC. As shown in Figure 1E‐H, increased expression of Snail correlated significantly with tumor size (P = .018), lymph node metastasis (P = .033), distant metastasis (P = .025), and clinical stage (P = .024). Moreover, high Snail expression was associated with poor prognosis of CRC patients (Figure 1I).
Figure 1.
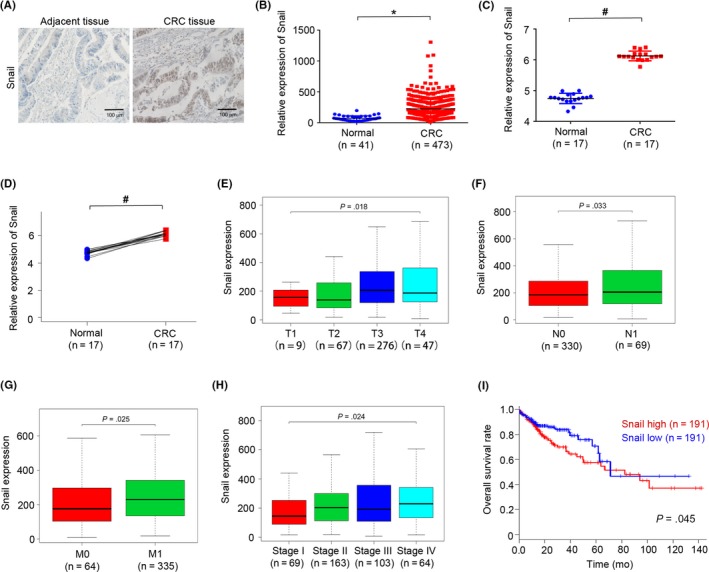
Snail is upregulated in colorectal cancer (CRC) and predicts poor survival of patients. A, Typical immunohistochemical staining of Snail in CRC tumor sample and adjacent tissue sample. B, Expression of Snail in 473 CRC cancer tissues and 41 normal tissues of patients from The Cancer Genome Atlas (TCGA) database. C, Expression of Snail in 17 CRC cancer tissues and 17 normal tissues of patients from the Gene Expression Omnibus (GEO) database (GSE32323). D, Expression of Snail in CRC cancer and its matched adjacent normal tissues of 17 patients from the GEO database (GSE32323). E, Association with Snail mRNA expression with tumor size in CRC patients in TCGA database. F, Association with Snail mRNA expression with lymph node metastasis in CRC patients in TCGA database. N0, no lymph node metastasis; N1, lymph node metastasis. G, Association with Snail mRNA expression with distant metastasis in CRC patients in TCGA database. M0, no distant metastasis; M1, distant metastasis. H, Relative mRNA expression of Snail in stage I, II, III, and IV CRC patients based on data available from TCGA. I, Impact of Snail mRNA expression on overall survival in CRC patients in TCGA. P < .05; # P < .01
3.2. Overexpression of Snail induces 5‐Fu resistance and increases ABCB1 expression in CRC cells
Emerging evidence shows that the EMT phenotype is associated with acquired chemoresistance in cancer cells.21 To explore the correlation between the EMT phenotype and drug resistance, we overexpressed Snail in CRC cells. As shown in Figure 2A,B, compared with the empty vector transfected with pcDNA3.1, the mRNA and protein expression levels of Snail were significantly increased in HCT116 and SW480 cells transfected with pcDNA‐Snail. Next, we investigated the relationship between Snail overexpression and chemoresistance in CRC cells. The results of a cell viability assay showed that overexpression of Snail contributed to 5‐Fu resistance in HCT116 and SW480 cells (Figure 2C). We further investigated the underlying mechanism of chemoresistance induction by Snail. Numerous processes, including ABC transporter‐mediated drug efflux, antiapoptosis, DNA damage checkpoint response, and DNA repair, are closely related to chemoresistance acquisition in cancer cells. The ABC transport proteins involved in chemoresistance include ABCB1, ABCC1/2/3/4/5/6/10/11/12, and ABCG2.16 The DNA repair enzymes, including ERCC1/2 and XRCC1, have shown to be involved in chemoresistance in cancers.22, 23, 24 Mcl‐1 is a protein involved in suppression of cell apoptosis. A study showed that Mcl‐1 can modulate drug resistance in cancer cells.25 DNA damage checkpoint response proteins, including BRCA1/2, MSH2/6, and GADD45α/β, have shown to be involved in chemoresistance in cancers.26, 27, 28, 29, 30 Both GST and TUBB3 can also modulate drug resistance in cancer cells.31, 32 To investigate the underlying mechanism of chemoresistance induction by Snail, we measured expression patterns of these chemoresistance‐related genes in Snail‐overexpressing CRC cells. The result of quantitative real‐time PCR (qRT‐PCR) showed that overexpression of Snail upregulated the mRNA expression of ABCB1 but had no significant effect on that of ABCC1/2/3/4/5/6/10/11/12, ABCG2, ERCC1/2, XRCC1, Mcl‐1, BRCA1/2, MSH2/6, GST, γ‐GT1, TUBB3, or GADD45α/β (Figure 2D). In addition, overexpression of Snail increased ABCB1 protein expression in both HCT116 and SW480 cells, but not ABCC1/2/3/12, ABCG2, or ERCC1 (Figure 2E). We also found that overexpression of Snail decreased the mRNA and protein expression of E‐cadherin, which is a hallmark of EMT (Figure 2D,E), suggesting that overexpression of Snail could induce the EMT in CRC cells.
Figure 2.
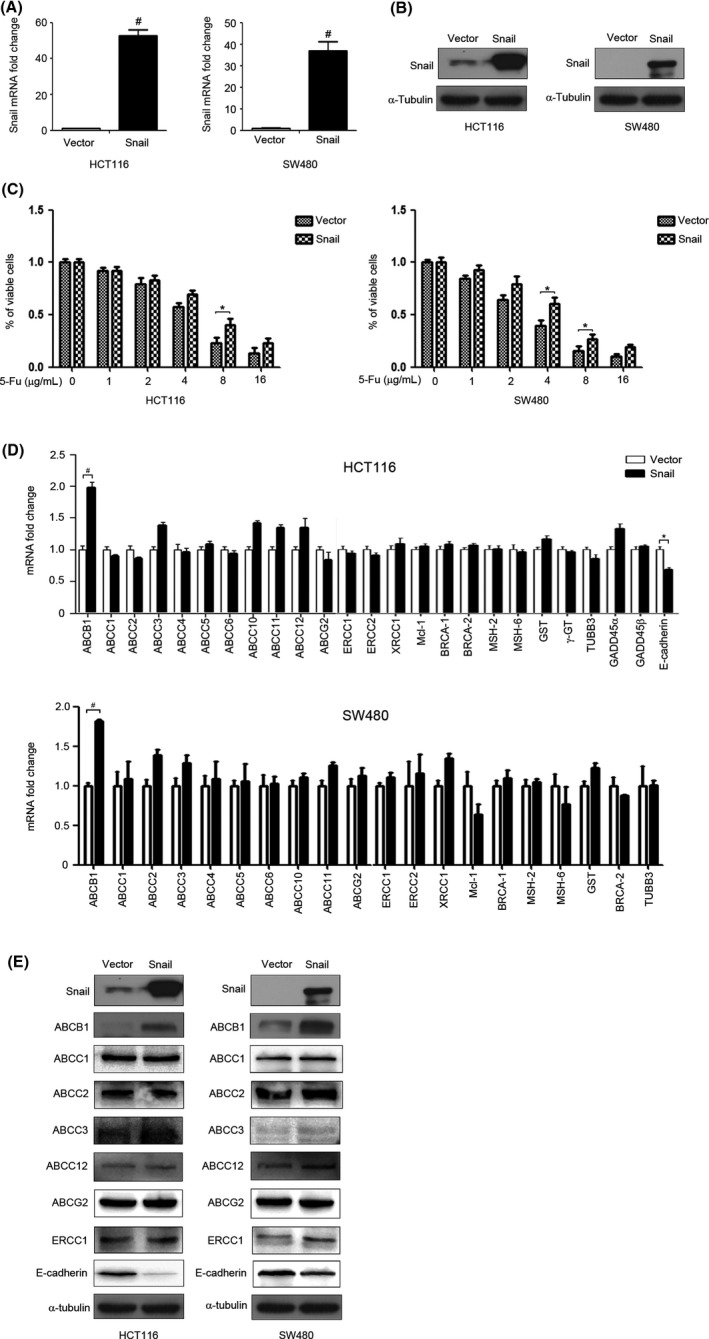
Overexpression of Snail induces 5‐fluorouracil (5‐Fu) resistance and increases ATP‐binding cassette subfamily B member 1 (ABCB1) expression in colorectal cancer cells. A, HCT116 and SW480 cells were transfected with pcDNA‐3.1 (Vector) or pcDNA‐Snail (Snail) for 48 h and Snail mRNA expression was detected by quantitative real‐time PCR (qRT‐PCR). B, HCT116 and SW480 cells were transfected with Vector or Snail for 48 h. Snail protein expression was detected by western blot analysis. α‐Tubulin served as the loading control. C, HCT116 and SW480 cells were transfected with Vector or Snail for 24 h and then treated with different concentrations of 5‐Fu for 48 h. The viability of cells was detected by CCK‐8 assay. D, HCT116 and SW480 cells were transfected with Vector or Snail for 48 h and the mRNA expression of drug resistance‐related genes were detected by qRT‐PCR. E, HCT116 and SW480 cells were transfected with Vector or Snail for 48 h. The protein expression levels of Snail, ABCB1, ABCC1, ABCC2, ABCC3, ABCG2, ABCC12, ERCC1, and E‐cadherin were detected by western blotting. α‐Tubulin served as the loading control.*P < .05; # P < .01
3.3. Snail‐mediated ABCB1 upregulation is independent of β‐catenin, Foxo3a, STAT3, PXR, and CAR
Next, we probed the molecular mechanism of Snail‐mediated ABCB1 upregulation. Several transcription factors, such as β‐catenin, Foxo3a, STAT3, PXR, and CAR, affect ABCB1 expression by directly binding to its promoter.33, 34, 35, 36, 37 Thus, we investigated whether Snail could influence their activities by detecting the mRNA and protein expression of these transcription factors by qRT‐PCR and western blot analysis. Unfortunately, we found that Snail had no significant effect on the mRNA and protein expression levels of β‐catenin, Foxo3a, STAT3, PXR, or CAR (Figure 3A,B), suggesting the presence of an alternative mechanism for Snail‐mediated ABCB1 upregulation.
Figure 3.
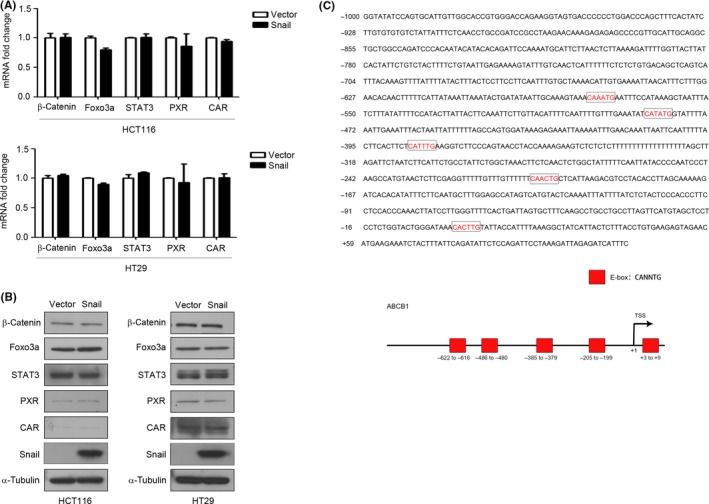
Snail has no effect on the expression of β‐catenin, Foxo3a, STAT3, PXR and CAR. A, HCT116 and HT29 cells were transfected with Vector or Snail for 48 h and the mRNA expression levels of β‐catenin, Foxo3a, STAT3, PXR, and CAR were detected by qRT‐PCR. B, HCT116 and HT29 cells were transfected with Vector or Snail for 48 h. Protein expression levels of β‐catenin, Foxo3a, STAT3, PXR, and CAR were detected by western blot analysis. α‐Tubulin served as the loading control. C, Human ATP‐binding cassette subfamily B member 1(ABCB1) promoter sequences were obtained from NCBI Map Viewer. Putative Snail‐binding sites (E‐box) are shown
3.4. Snail directly regulates ABCB1 transcription
We envisioned that Snail, as a transcription factor, might directly regulate ABCB1 transcription. To confirm the hypothesis, we searched the –1000 to +180 bp region of the ABCB1 promoter and analyzed the possible binding sites (E‐box, consensus sequence: CANNTG, N, random bases). There were 5 possible Snail‐binding sites in the promoter region of the ABCB1 gene (Figure 3C). We first constructed 5 promoter reporter plasmids (ABCB1 LucA, ABCB1 LucB, ABCB1 LucC, ABCB1 LucD, and ABCB1 LucE), which contained different lengths of the ABCB1 promoter (Figure 4A). There were 5 E‐boxes in ABCB1 LucA, 4 in ABCB1 LucB, 3 in ABCB1 LucC, 2 in ABCB1 LucD, and 1 in ABCB1 LucE. The subsequent dual‐luciferase reporter assay illustrated that overexpression of Snail activated ABCB1 LucA but not the other constructs (Figure 4A). To further identify the most crucial sites for Snail binding, we carried out site‐directed mutagenesis to construct a promoter reporter plasmid with an E‐box mutation (ABCB1‐LucA mE1). The subsequent dual‐luciferase reporter assay detected decreased activity of ABCB1‐LucA mE1 compared with that of ABCB1‐LucA (Figure 4B), indicating that mutation of the E‐box inhibited Snail‐mediated activation of the ABCB1 promoter. Finally, the ChIP assay verified that Snail was capable of directly binding to the promoter region of the ABCB1 gene (Figure 4C) and ChIP‐qPCR was used to quantify the binding affinity of Snail (Figure 4D). Collectively, these results revealed that Snail promoted ABCB1 expression by directly binding to its promoter region and that the E‐box1 motif in the regulatory region played an important role in the promoter activation.
Figure 4.
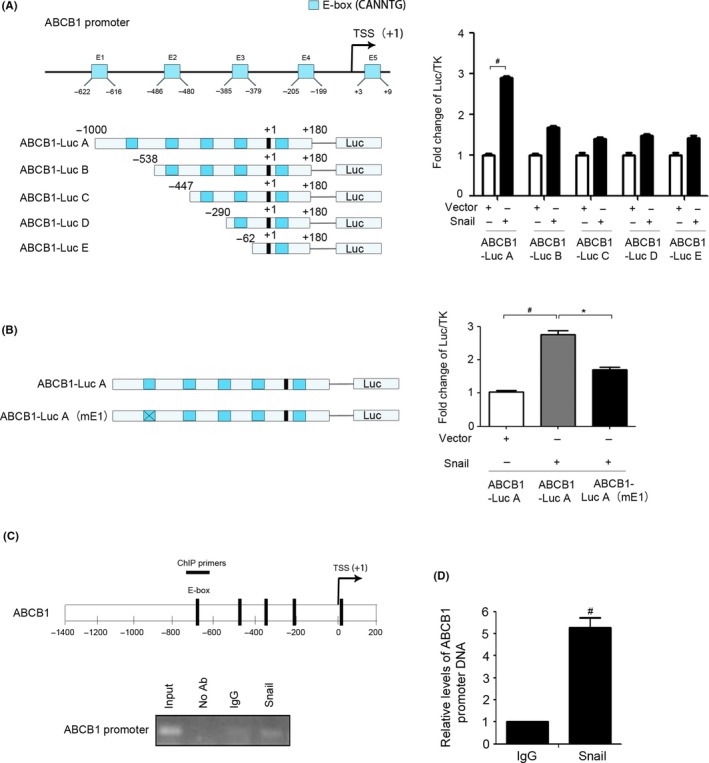
Direct regulation of ATP‐binding cassette subfamily B member 1 (ABCB1) by Snail. A, Left, a series of deletion mutants of the ABCB1 promoter. TSS, transcription start site. Right, ABCB1‐LucA, ABCB1‐LucB, ABCB1‐LucC, ABCB1‐LucD, and ABCB1‐LucE were transfected into HEK293T cells with or without Vector and Snail for 48 h. The cells were subjected to the luciferase reporter assay as described. B, Left, WT and mutated E‐box in the ABCB1 promoter. Right, ABCB1‐LucA and ABCB1‐LucA (mE1) were transfected into HEK293T cells with or without Vector and Snail for 48 h. Cells were subjected to the luciferase reporter assay as described. C, ChIP assay was carried out using anti‐Snail Ab or irrelevant anti‐IgG Ab as a negative control. The protein‐chromatin immunoprecipitates were subjected to PCR analysis as described. D, ChIP assay was carried out using anti‐Snail Ab or irrelevant anti‐IgG Ab as a negative control. The chromatin was subjected to quantitative PCR analysis. *P < .05; # P < .01
3.5. ABCB1 is crucial for Snail‐mediated 5‐Fu resistance in CRC cells
We further investigated the role of ABCB1 in the Snail‐mediated chemoresistance of CRC cells to 5‐Fu. After transfection of HCT116 cells with si‐ABCB1 and si‐negative control, the expression of ABCB1 was detected by western blot analysis and RT‐PCR. The results showed clear suppression of ABCB1 expression in HCT116 cells transfected with si‐ABCB1 (Figure 5A,B). A cell viability assay was then performed to elucidate the role of ABCB1 in Snail‐mediated chemoresistance to 5‐Fu. As shown in Figure 5C, inhibition of ABCB1 expression partially suppressed Snail‐mediated chemoresistance of HCT116 cells to 5‐Fu. We further investigated the effect of chemotherapy on the expression of Snail and ABCB1 in HCT116 cells. The results showed that oxaliplatin treatment significantly increased the mRNA and protein expression of Snail and ABCB1 (Figure 5D,E). Moreover, the mRNA and protein expression of Snail and ABCB1 was enhanced in the 5‐Fu‐resistant CRC cell line HCT‐8/5‐Fu compared with HCT‐8 cells (Figure 5F,G). The cell viability assay was undertaken on HCT8 cells, HCT8/5‐Fu cells, and HCT8/5‐Fu cells transfected with ABCB1 siRNA. The results showed that HCT8/5‐Fu cells displayed 5‐Fu resistance compared with HCT8 cells and inhibition of ABCB1 partly reversed the resistance of HCT8/5‐Fu cells to 5‐Fu (Figure S2). Suppression of Snail clearly decreased the mRNA and protein expression of ABCB1 in HCT‐8/5‐Fu cells (Figure 5H,I). These results suggested that Snail overexpression promoted ABCB1 expression and thus led to 5‐Fu resistance in CRC cells.
Figure 5.
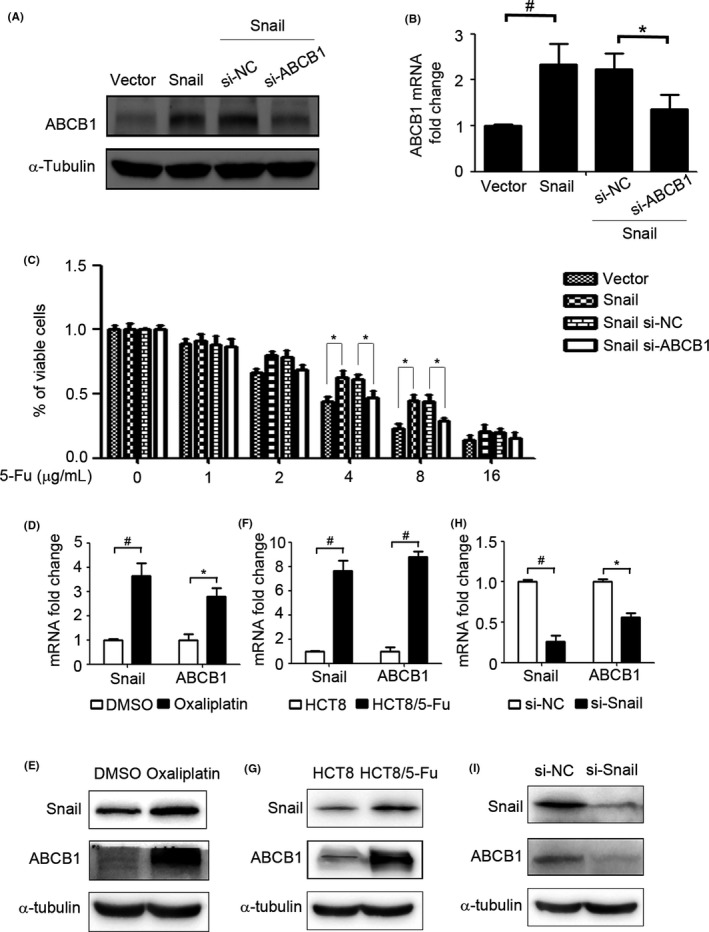
ATP‐binding cassette subfamily B member 1 (ABCB1) is crucial for Snail‐mediated 5‐fluorouracil (5‐Fu) resistance. A,B, HCT116 cells were transfected with si‐negative (si‐NC) or si‐ABCB1 with Vector or Snail for 48 h. The protein expression and mRNA expression of ABCB1 were detected by western blot analysis and RT‐PCR. C, HCT116 cells were transfected with si‐negative (si‐NC) or si‐ABCB1 with Vector or Snail for 48 h. Cell viability was determined by CCK‐8 assay. D,E, HCT116 cells were treated with oxaliplatin (5 μg/mL) for 48 h and the mRNA (D) and protein (E) expression levels of Snail and ABCB1 were detected by RT‐PCR and western blot analysis, respectively. F,G, mRNA (F) and protein (G) expression levels of Snail and ABCB1 in HCT‐8 and HCT‐8/5‐Fu were detected by RT‐PCR and western blot analysis, respectively. H,I, HCT‐8/5‐Fu cells were transfected with si‐NC or si‐Snail for 48 h. mRNA (H) and protein (I) expression levels of Snail and ABCB1 were detected by RT‐PCR and western blot analysis, respectively. α‐Tubulin served as the loading control.*P < .05; # P < .01
3.6. Expression of Snail and ABCB1 are significantly relative in CRC patients
Accumulating reports show that patients with Snail‐overexpressing CRC are more inclined to develop distant metastases and have a poor response to chemotherapy.38, 39 Similarly, abundant ABCB1 expression is associated with CRC prognosis.20 To determine the expression of Snail and ABCB1 in CRC patients and their contribution to chemoresistance, we used immunohistochemistry to investigate the expression of Snail and ABCB1 in 46 tumor samples from CRC patients. Two representative cases are shown in Figure 6A. In CRC tumor samples, Snail expression was evident in 29 cases (63.04%) and ABCB1 expression was seen in 24 cases (52.17%). There was a clear correlation between Snail and ABCB1 expression (Figure 6B). Moreover, we analyzed the GEO database and found that the expression of Snail was positively correlated with the ABCB1 mRNA in 211 CRC patients (Figure 6C). The results from TCGA patient data further confirmed the coexpression of Snail and ABCB1 (Figure 6D). We then investigate the expression of ABCB1 in CRC tumor samples and normal tissues. We analyzed the GEO patient database and found that ABCB1 mRNA expression was higher in CRC tissues than in normal tissues (Figure 6E). Moreover, high ABCB1 expression was associated with poor prognosis of CRC patients (Figure 6F). Collectively, these results suggested that activation of the Snail/ABCB1 axis promotes chemoresistance.
Figure 6.
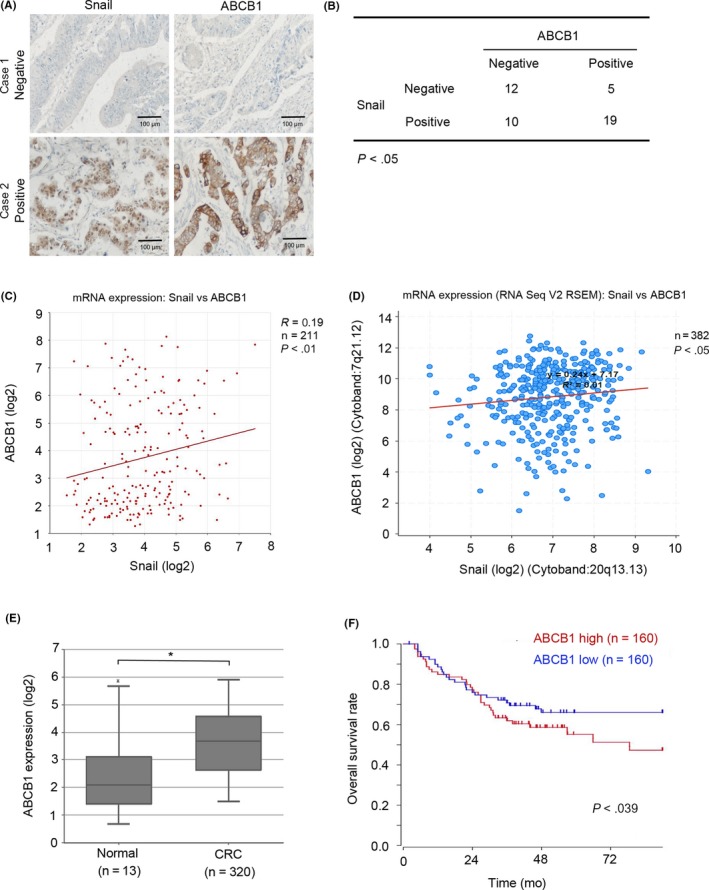
Expression of Snail and ATP‐binding cassette subfamily B member 1 (ABCB1) are significantly relative in colorectal cancer (CRC) patients. A, Immunohistochemical staining of Snail and ABCB1 in 2 representative CRC tumor samples and matched normal epithelium samples that are negative for Snail and ABCB1 expression (case 1) or show coexpression of Snail and ABCB1 (case 2). B, Immunohistochemical staining of Snail and ABCB1 in 46 CRC tumor samples. Positive and negative expression levels of Snail and ABCB1 were counted and analyzed. C,D, Pearson’s correlation between Snail and ABCB1 in 211 CRC tissues from GSE75315 (C) and 382 CRC tissues from The Cancer Genome Atlas database (D). E, Expression of ABCB1 in 320 CRC cancer tissues and 13 normal tissues of patients from the Gene Expression Omnibus (GEO) database (GSE24551). F, Impact of ABCB1 mRNA expression on overall survival in CRC patients in the GEO database (GSE24551). *P < .05
4. DISCUSSION
Increasing evidence shows that the EMT, which endows cancer cells with invasive and metastatic characteristics, is associated with acquired malignant phenotypes, such as induction of cancer stem‐like features,40 radiotherapy and chemotherapy resistance,41, 42, 43 immunosuppression,1 and angiogenesis.44, 45 The pleiotropic activations triggered by EMT controllers give rise to complex behavior of cancer cells, which could be responsible for the difficulties encountered during treatment for metastatic cancers. However, very few studies have investigated the underlying mechanisms of EMT‐mediated chemoresistance.
Herein, we found that the transcription factor Snail, which mediates the EMT, also regulates the expression of the multidrug‐resistant transporter protein ABCB1. In particular, we identify that ABCB1 plays an important role in Snail‐mediated chemoresistance in CRC cells based on the following results: (i) overexpression of Snail promoted ABCB1 expression but not that of other chemoresistance‐related genes; (ii) Snail directly regulated ABCB1 transcription by binding to its promoter; (iii) inhibition of ABCB1 attenuated Snail‐induced 5‐Fu resistance; (iv) inhibition of Snail decreased ABCB1 in HCT‐8/5‐Fu cells; and (v) coexpression of Snail and ABCB1 increased the risk of 5‐Fu resistance in CRC patients. To our knowledge, this study is the first to confirm that the resistance‐related gene ABCB1 is a direct target of Snail.
We previously found that cisplatin‐resistant lung cancer cells show EMT and cancer stem‐like properties, with Snail playing an important role in maintaining the aggressive phenotype.12 In this study, we further investigated the underlying molecular mechanisms of Snail‐induced chemoresistance. Twenty‐four drug resistance‐related genes were examined in this study, including the ABC transport proteins ABCB1, ABCC1/2/3/4/5/6/10/11/12, and ABCG2, the DNA repair proteins ERCC1/2 and XRCC1, the antiapoptosis protein Mcl‐1, and the DNA damage checkpoint proteins BRCA1/2 and MSH2/6. We found that Snail upregulated ABCB1 and not the other genes, suggesting that the other genes are not regulated by Snail in CRC cells. Some of these genes have possible Snail‐binding sites in their promoter region, such as ERCC1 and ABCC4. Research has shown that Snail induces cisplatin resistance in head and neck squamous cell carcinoma by upregulation of ERCC1.22 More recently, a study reported that Twist increases the expression of ABCC4 and ABCC5 in breast cancer cells.46 However, none of these genes were affected by the overexpression of Snail in CRC cells in this study, suggesting the diversity and complexity of chemoresistance in different cancer cells. Knockdown of ABCB1 further indicated that ABCB1 plays an important role in Snail‐induced chemoresistance. Importantly, oxaliplatin treatment increased Snail and ABCB1 expression in CRC cells. Based on these results, and in light of our previous findings, it is hypothesized that initial chemotherapy leads to the apoptosis of most epithelial‐like cancer cells whereas the mesenchymal‐like cancer cells evade treatment due to the upregulation of Snail and the resulting overexpression of ABCB1, which not only triggers chemoresistance, but also induces cancer stem‐like characteristics. This explains why cancer cells initially sensitive to chemotherapy gradually display a multidrug‐resistant and aggressive phenotype.
The ABC family‐mediated efflux results in multidrug resistance by pumping chemotherapeutic drugs out of cancer cells.16 The most important member of the ABC transporter protein family, ABCB1, is linked to various factors that induce multidrug resistance. Despite the well‐defined role of ABCB1 in chemoresistance, most previous studies have focused on gene polymorphisms and mutations in ABCB1 while ignoring its regulatory mechanisms.47, 48, 49 Additionally, a few transcription factors have been reported to directly interact with the promoter of ABCB1 and induce its expression, such as β‐catenin, Foxo3a, STAT3, PXR, and CAR.33, 34, 35, 37 However, in the present study, we found that none of these transcription factors were affected by Snail. Importantly, we found that Snail directly regulates ABCB1 transcription by binding to the E‐box motif in the promoter of ABCB1. Given that the SANG domain has been reported to be involved in transcriptional regulation of the target gene of Snail,50, 51 it will be valuable to further investigate the role of the SANG domain in ABCB1 transcription regulation.
Our study revealed that, beyond invasion and metastasis, the EMT regulator Snail could induce chemoresistance by directly upregulating the ABC transporter ABCB1. Our results provide a better understanding of the molecular mechanism underlying cancer metastasis and chemoresistance. The Snail/ABCB1 axis could be a potential CRC therapeutic target for simultaneously addressing cancer metastasis and chemoresistance.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
ACKNOWLEDGEMENTS
This work was funded by the National Natural Science Foundation of China (No. 81502599), the Natural Science Foundation of Anhui Province (No. 1608085QH217), China Postdoctoral Science Foundation‐funded project (No. 2016M592040), Anhui Province Postdoctoral Science Foundation‐funded project (No. 2016B142), the Key Projects of Natural Science Research of Anhui University of Chinese Medicine (2017zrzd012), and Hunan Young Talent, China (No. 2017RS3051).
Wang H, Li J‐M, Wei W, et al. Regulation of ATP‐binding cassette subfamily B member 1 by Snail contributes to chemoresistance in colorectal cancer. Cancer Sci. 2020;111:84–97. 10.1111/cas.14253
Contributor Information
Xiao‐Dong Ma, Email: o-omaxiaodong@163.com.
Guan‐Min Jiang, Email: jguanmin@163.com.
Bao‐Long Wang, Email: wbl196555@163.com.
REFERENCES
- 1. Thiery JP, Acloque H, Huang RY, et al. Epithelial‐mesenchymal transitions in development and disease. Cell. 2009;139:871‐890. [DOI] [PubMed] [Google Scholar]
- 2. Qin H, Liu X, Li F, et al. PAD1 promotes epithelial‐mesenchymal transition and metastasis in triple‐negative breast cancer cells by regulating MEK1‐ERK1/2‐MMP2 signaling. Cancer Lett. 2017;409:30‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liang X. EMT: new signals from the invasive front. Oral Oncol. 2011;47:686‐687. [DOI] [PubMed] [Google Scholar]
- 4. Qureshi R, Arora H, Rizvi MA. EMT in cervical cancer: its role in tumour progression and response to therapy. Cancer Lett. 2015;356:321‐331. [DOI] [PubMed] [Google Scholar]
- 5. Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415‐428. [DOI] [PubMed] [Google Scholar]
- 6. Pearlman RL, Montes de Oca MK, Pal HC, et al. Potential therapeutic targets of epithelial‐mesenchymal transition in melanoma. Cancer Lett. 2017;391:125‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang H, Wang HS, Zhou BH, et al. Epithelial‐mesenchymal transition (EMT) induced by TNF‐alpha requires AKT/GSK‐3beta‐mediated stabilization of snail in colorectal cancer. PLoS ONE. 2013;8:e56664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deng JJ, Zhang W, Xu XM, et al. Twist mediates an aggressive phenotype in human colorectal cancer cells. Int J Oncol. 2016;48:1117‐1124. [DOI] [PubMed] [Google Scholar]
- 9. Cheng GZ, Chan J, Wang Q, et al. Twist transcriptionally up‐regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 2007;67:1979‐1987. [DOI] [PubMed] [Google Scholar]
- 10. Arumugam T, Ramachandran V, Fournier KF, et al. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69:5820‐5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maseki S, Ijichi K, Tanaka H, et al. Acquisition of EMT phenotype in the gefitinib‐resistant cells of a head and neck squamous cell carcinoma cell line through Akt/GSK‐3beta/snail signalling pathway. Brit J Cancer. 2012;106:1196‐1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang H, Zhang G, Zhang H, et al. Acquisition of epithelial‐mesenchymal transition phenotype and cancer stem cell‐like properties in cisplatin‐resistant lung cancer cells through AKT/beta‐catenin/Snail signaling pathway. Eur J Pharmacol. 2014;723:156‐166. [DOI] [PubMed] [Google Scholar]
- 13. He H, Ni J, Huang J. Molecular mechanisms of chemoresistance in osteosarcoma (Review). Oncol Lett. 2014;7:1352‐1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zahreddine H, Borden KL. Mechanisms and insights into drug resistance in cancer. Front Pharmacol. 2013;4:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Assaraf YG. Molecular basis of antifolate resistance. Cancer Metast Rev. 2007;26:153‐181. [DOI] [PubMed] [Google Scholar]
- 16. Fukuda Y, Schuetz JD. ABC transporters and their role in nucleoside and nucleotide drug resistance. Biochem Pharmacol. 2012;83:1073‐1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nemethova V, Razga F. Overexpression of ABCB1 as prediction marker for CML: how close we are to translation into clinics? Leukemia. 2017;31:266‐267. [DOI] [PubMed] [Google Scholar]
- 18. Sharom FJ. The P‐glycoprotein multidrug transporter. Essays Biochem. 2011;50:161‐178. [DOI] [PubMed] [Google Scholar]
- 19. Andersen V, Svenningsen K, Knudsen LA, et al. Novel understanding of ABC transporters ABCB1/MDR/P‐glycoprotein, ABCC2/MRP2, and ABCG2/BCRP in colorectal pathophysiology. World J Gastroentero. 2015;21:11862‐11876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gayoung L, Joung JY, Cho JH, et al. Overcoming P‐glycoprotein‐mediated multidrug resistance in colorectal cancer: potential reversal agents among herbal medicines. Evid Based Complement Alternat Med. 2018;2018:3412074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Garg M. Epithelial plasticity and cancer stem cells: major mechanisms of cancer pathogenesis and therapy resistance. World J Stem Cell. 2017;9:118‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hsu DS, Lan HY, Huang CH, et al. Regulation of excision repair cross‐complementation group 1 by Snail contributes to cisplatin resistance in head and neck cancer. Clin Cancer Res. 2010;16:4561‐4571. [DOI] [PubMed] [Google Scholar]
- 23. Biason P, Hattinger CM, Innocenti F, et al. Nucleotide excision repair gene variants and association with survival in osteosarcoma patients treated with neoadjuvant chemotherapy. Pharmacogenomics J. 2012;12:476‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang Z, Sun C, Zhang L, et al. Triptolide interferes with XRCC1/PARP1‐mediated DNA repair and confers sensitization of triple‐negative breast cancer cells to cisplatin. Biomed Pharmacother. 2019;109:1541‐1546. [DOI] [PubMed] [Google Scholar]
- 25. Maji S, Shriwas O, Samal SK, et al. STAT3‐ and GSK3beta‐mediated Mcl‐1 regulation modulates TPF resistance in oral squamous cell carcinoma. Carcinogenesis. 2019;40:173‐183. [DOI] [PubMed] [Google Scholar]
- 26. Xiao W, Xiong Z, Yuan C, et al. Low neighbor of Brca1 gene expression predicts poor clinical outcome and resistance of sunitinib in clear cell renal cell carcinoma. Oncotarget. 2017;8:94819‐94833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang W, Figg WD. Secondary BRCA1 and BRCA2 alterations and acquired chemoresistance. Cancer Biol Ther. 2008;7:1004‐1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ting S, Mairinger FD, Hager T, et al. ERCC1, MLH1, MSH2, MSH6, and betaIII‐tubulin: resistance proteins associated with response and outcome to platinum‐based chemotherapy in malignant pleural mesothelioma. Clin Lung Cancer. 2013;14:558‐567. [DOI] [PubMed] [Google Scholar]
- 29. Chiou SK, Hodges A, Hoa N, et al. Suppression of growth arrest and DNA damage‐inducible 45alpha expression confers resistance to sulindac and indomethacin‐induced gastric mucosal injury. J Pharmacol Exp Ther. 2010;334:693‐702. [DOI] [PubMed] [Google Scholar]
- 30. Hou XJ, Zhao QD, Jing YY, et al. Methylation mediated Gadd45beta enhanced the chemosensitivity of hepatocellular carcinoma by inhibiting the stemness of liver cancer cells. Cell Biosci. 2017;7:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sekino Y, Han X, Kawaguchi T, et al. TUBB3 Reverses Resistance to Docetaxel and Cabazitaxel in Prostate Cancer. Int J Mol Sci. 2019;20:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang B, Shen C, Li Y, et al. Oridonin overcomes the gemcitabine resistant PANC‐1/Gem cells by regulating GST pi and LRP/1 ERK/JNK signalling. Onco Targets Ther. 2019;12:5751‐5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang X, Xiao W, Wang L, et al. Deactivation of signal transducer and activator of transcription 3 reverses chemotherapeutics resistance of leukemia cells via down‐regulating P‐gp. PLoS ONE. 2011;6:e20965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang H, Zhang X, Wu X, et al. Interference of Frizzled 1 (FZD1) reverses multidrug resistance in breast cancer cells through the Wnt/beta‐catenin pathway. Cancer Lett. 2012;323:106‐113. [DOI] [PubMed] [Google Scholar]
- 35. Hui RC, Francis RE, Guest SK, et al. Doxorubicin activates FOXO3a to induce the expression of multidrug resistance gene ABCB1 (MDR1) in K562 leukemic cells. Mol Cancer Ther. 2008;7:670‐678. [DOI] [PubMed] [Google Scholar]
- 36. Maglich JM, Stoltz CM, Goodwin B, et al. Nuclear pregnane x receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol Pharmacol. 2002;62:638‐646. [DOI] [PubMed] [Google Scholar]
- 37. Wei P, Zhang J, Dowhan DH, et al. Specific and overlapping functions of the nuclear hormone receptors CAR and PXR in xenobiotic response. Pharmacogenomics J. 2002;2:117‐126. [DOI] [PubMed] [Google Scholar]
- 38. Palmer HG, Larriba MJ, Garcia JM, et al. The transcription factor SNAIL represses vitamin D receptor expression and responsiveness in human colon cancer. Nat Med. 2004;10:917‐919. [DOI] [PubMed] [Google Scholar]
- 39. Pena C, Garcia JM, Silva J, et al. E‐cadherin and vitamin D receptor regulation by SNAIL and ZEB1 in colon cancer: clinicopathological correlations. Hum Mol Genet. 2005;14:3361‐3370. [DOI] [PubMed] [Google Scholar]
- 40. Mani SA, Guo W, Liao MJ, et al. The epithelial‐mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704‐715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang AD, Fan F, Camp ER, et al. Chronic oxaliplatin resistance induces epithelial‐to‐mesenchymal transition in colorectal cancer cell lines. Clin Cancer Res. 2006;12:4147‐4153. [DOI] [PubMed] [Google Scholar]
- 42. Kurrey NK, Jalgaonkar SP, Joglekar AV, et al. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53‐mediated apoptosis and acquiring a stem‐like phenotype in ovarian cancer cells. Stem Cell. 2009;27:2059‐2068. [DOI] [PubMed] [Google Scholar]
- 43. Gungor C, Zander H, Effenberger KE, et al. Notch signaling activated by replication stress‐induced expression of midkine drives epithelial‐mesenchymal transition and chemoresistance in pancreatic cancer. Cancer Res. 2011;71:5009‐5019. [DOI] [PubMed] [Google Scholar]
- 44. Mironchik Y, Winnard PT, Vesuna F, et al. Twist overexpression induces in vivo angiogenesis and correlates with chromosomal instability in breast cancer. Cancer Res. 2005;65:10801‐10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sun T, Zhao N, Zhao XL, et al. Expression and functional significance of Twist1 in hepatocellular carcinoma: its role in vasculogenic mimicry. Hepatology. 2010;51:545‐556. [DOI] [PubMed] [Google Scholar]
- 46. Saxena M, Stephens MA, Pathak H, et al. Transcription factors that mediate epithelial‐mesenchymal transition lead to multidrug resistance by upregulating ABC transporters. Cell Death Dis. 2011;2:e179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang Y, Yang X, Shi J, et al. Combination analysis of NOS3, ABCB1 and IL23R polymorphisms with alcohol‐induced osteonecrosis of the femoral head risk in Chinese males. Oncotarget. 2017;8:33770‐33778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kwon WS, Rha SY, Jeung HC, et al. G‐T haplotype (2677G>T/A and 3435C>T) of ABCB1 gene polymorphisms is associated with ethnic differences to paclitaxel sensitivity in cancer cells with different gene expression pattern. Cancer Lett. 2009;277:155‐163. [DOI] [PubMed] [Google Scholar]
- 49. Tandia M, Mhiri A, Paule B, et al. Correlation between clinical response to sorafenib in hepatocellular carcinoma treatment and polymorphisms of P‐glycoprotein (ABCB1) and of breast cancer resistance protein (ABCG2): monocentric study. Cancer Chemother Pharm. 2017;79:759‐766. [DOI] [PubMed] [Google Scholar]
- 50. Peinado H, Ballestar E, Esteller M, et al. Snail mediates E‐cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol. 2004;24:306‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hou Z, Peng H, Ayyanathan K, et al. The LIM protein AJUBA recruits protein arginine methyltransferase 5 to mediate SNAIL‐dependent transcriptional repression. Mol Cell Biol. 2008;28:3198‐3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


