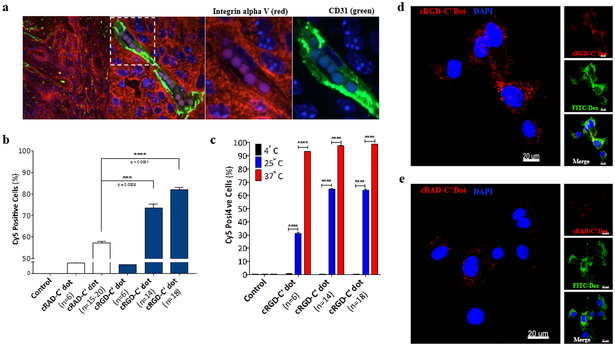
Figure 2. cRGD-C’ dots demonstrate a ligand-dependent increase in cellular binding and uptake in αν integrin-expressing primary cells from RCAS/tv-a glioblastoma.
a) Immunofluorescence staining of tissue harvested from an RCAS/tv-a tumor bearing mouse. Integrin αν expression (red), is demonstrated throughout the tumor and along endothelial cells lining its neovasculature. Tumor neovasculature was visualized by anti-CD31 (green) antibody. Cell nuclei are counterstained with DAPI (blue). b) Comparison of cRGD-C’ dot and cRAD-C’ dot cell binding by flow cytometry. RCAS/tv-a derived glioma cells were incubated with cRAD-C’ dots or cRGD-C’ dots with varying ligand densities (n=6-20 and n=6-18, respectively) and analyzed (Student’s T-test, *** p = 0.0008, **** p < 0.0001). c) Temperature-dependent uptake of cRGD-C’ dot nanoparticles in RCAS/tv-a derived glioma cells. cRGD-C’ dot particles displaying 6, 14, and 18 cRGD per particle were incubated with glioma cells at 4, 25, and 37°C for 4 hours. Cell uptake was measured using flow cytometry (Student’s T-Test, **** p < 0.0001). d & e) High-resolution confocal microscopy of cRGD- and cRAD-C’ dot (n=18) uptake in glioma cells, respectively. Cells were treated with 100 nM cRGD-C’ dots (d) or cRAD-C’ dots (e) (red) in the presence of 1 mg/ml FITC-labeled 70kDa dextran (FITC-Dextran, green) for 4 hours. Treated cells were then fixed, counterstained with DAPI (blue). (Scale bars = 20 μm)