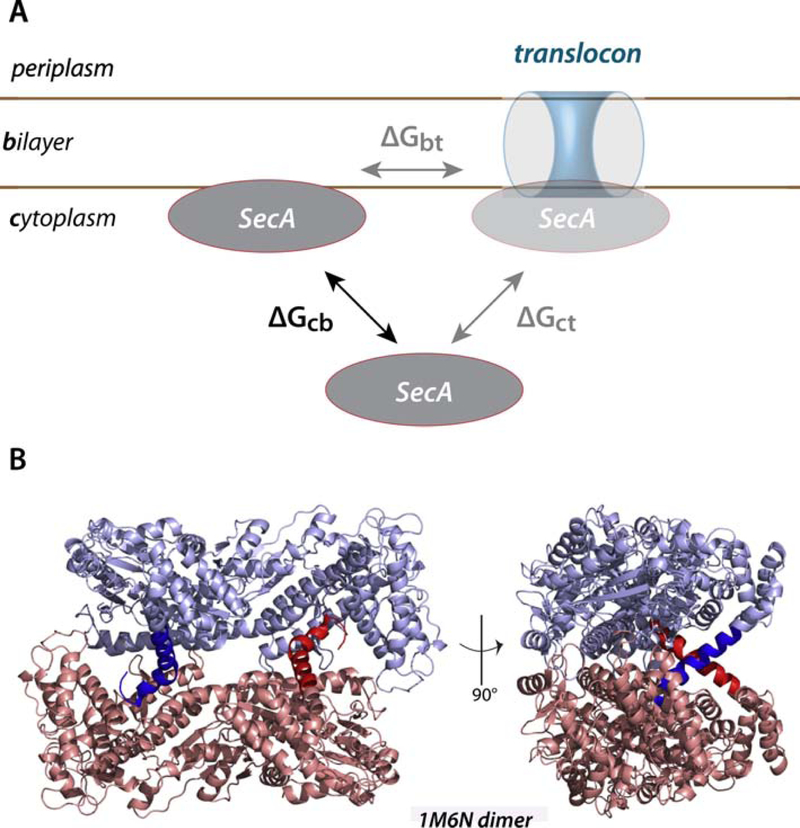
Figure 1. A thermodynamic scheme for the interaction of SecA with the SecYEG translocon and membrane lipid bilayer.
(panel A) and the molecular structure of the SecA dimer (PDB 1M6N) (panel B). SecA is known to bind to both negatively charged lipid bilayers and SecYEG translocon, but the relationship between the binding events is uncertain. We suggest that the relationship can be clarified by determining the relative free energies of transfer of SecA from the cytoplasm to the bilayer (ΔGcb) and to the translocon (ΔGct). The difference between these free energies(ΔGbt) should provide insights into the role of the lipid bilayer in the binding of SecA to the translocon. The general belief is that cytoplasmic SecA exists as a dimer represented by 1M6N. In the images in panel B, the monomeric protomers are colored blue and pink. The 21-residue N-terminal helices, highlighted by dark blue and dark red, are thought to be responsible for binding SecA to the bilayer. Note that the conformations of these helices suggest that they are also important in dimer formation.