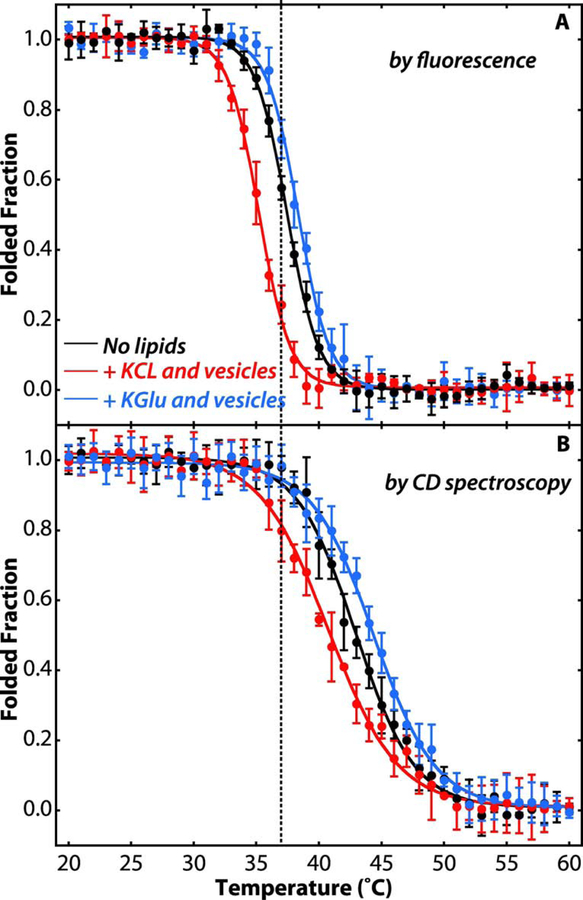
Figure 4. Potassium glutamate stabilizes SecA on the membrane.
(A) Temperature dependence of the unfolding of SecA tertiary structure as indicated by Trp fluorescence monitored at 340 nm (excitation at 295 nm). (B) Temperature dependence of the unfolding of SecA secondary structure as indicated molar ellipticity at 222 nm. Folded fractions were determined by assuming SecA (4 μM) was fully folded in its native state at 20°C and fully unfolded at 60°C. The folded fraction was determined from either the Trp fluorescence at 340 nm or the molar ellipticity at 222 nm, normalized by setting the intensities of the native and fully unfolded SecA to be 1 and 0 after correction for the temperature dependence of the fluorescence intensity. Black curves were determined for SecA in 100 mM KCl in the absence of lipid vesicles. The red curves show the thermal unfolding in 100 mM KCl and LUVs formed from E. coli lipids (4 mM). Blue curves are for 100 mM KGlu and LUVs formed from E. coli lipids (4 mM). When vesicles are present, approximately 95–98% of SecA is partitioned into them (see Figure 2). In KCl solutions, SecA bound to LUVs is less stable, as indicated by the shift of the curves to lower temperatures (red). In KGlu solutions, on the other hand, the stability of LUV-bound SecA is increased, as indicated by shifts of the unfolding curves to higher temperatures (blue). Experiments were carried out in triplicate. The error bars represent the standard errors of the mean (SEM) resulting from the three independent measurements.