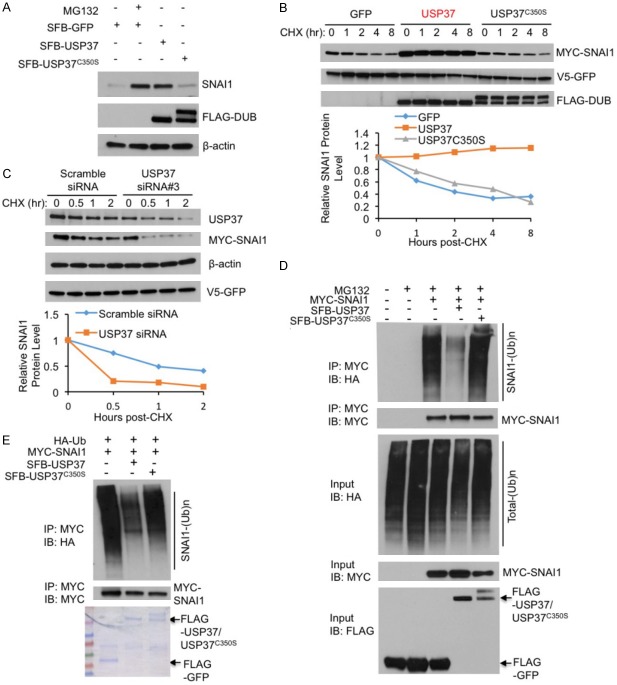
Figure 2.
USP37 stabilizes SNAI1 protein through deubiquitination. A. HEK293T cells were transfected with SFB-GFP, SFB-USP37, or SFB-USP37C350S, treated with 10 μM MG132 for 6 hours, harvested, and immunoblotted with antibodies against SNAI1, FLAG, and β-actin. B. Upper panel: HEK293T cells were co-transfected with V5-GFP and SFB-tagged GFP, USP37, or USP37C350S, treated with 100 μg ml-1 cycloheximide (CHX), harvested at different time points, and then immunoblotted with antibodies against MYC, V5, and FLAG. V5-GFP served as the control for transfection. Lower panel: quantification of SNAI1 protein levels (normalized to V5-GFP). C. Upper panel: HEK293T cells were co-transfected with MYC-SNAI1, V5-GFP and USP37 siRNA #3 or scrambled siRNA, treated with 100 μg ml-1 cycloheximide (CHX), harvested at different time points, and then immunoblotted with antibodies against MYC, V5, β-actin and FLAG. V5-GFP serves as the control for transfection. Lower panel: quantification of SNAI1 protein levels (normalized to V5-GFP). D. HEK293T cells were co-transfected with MYC-SNAI1, HA-ubiquitin (Ub), and SFB-tagged USP37 or USP37C350S, followed by immunoprecipitation with anti-MYC beads and immunoblotting with antibodies against HA and MYC. Cells were treated with 10 μM MG132 for 6 hours. Before immunoprecipitation, lysates were heated at 95°C for 5 minutes in the presence of 1% SDS (for denaturing), followed by 10-fold dilution with lysis buffer and sonication. E. SFB-GFP, SFB-USP37, and SFB-USP37C350S were purified from HEK293T cells transfected with SFB-tagged GFP or DUBs. Ubiquitinated MYC-SNAI1 was purified with anti-MYC beads from HEK293T cells co-transfected with MYC-SNAI1 and HA-Ub, and was then incubated with purified SFB-tagged GFP or DUBs. After the in vitro deubiquitination, bound proteins were eluted and immunoblotted with antibodies against HA and MYC. Purified proteins were analyzed by SDS-PAGE and Coomassie blue staining.