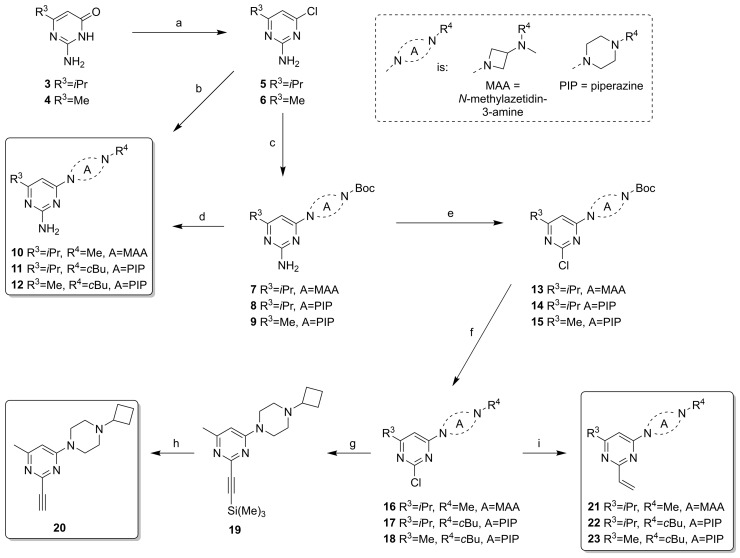
Scheme 1.
Synthesis of pyrimidine-based covalent ligands. (a) POCl3, reflux, 3 h, 26–33%; (b) N,N-dimethylazetidin-3-amine or 1-cyclobutylpiperazine, N,N-Diisopropylethylamine (DIPEA), dioxane, 150 °C, 30 min, μW, 59–65%; (c) t-butyl azetidin-3-yl(methyl)carbamate or t-butyl piperazine-1-carbamate, DIPEA, dioxane, 150 °C, 30 min, μW, 50–85%, 8: used without full purification; (d) 1. HCl, dioxane, rt, 2 h, 2. cyclobutanone, NaBH(OAc)3, dichloromethane (DCM), rt, overnight, 33% (two steps); (e) tBuONO or iPeONO, SbCl3, DCM, rt, 3 h, 23–40%; (f) 1. HCl, dioxane, rt, 2 h-overnight, 2. H2CO or cyclobutanone, NaBH(OAc)3, DCM, rt, overnight, 56–73% (two steps); (g) ethynyltrimethylsilane, CuI, Pd(dppf)Cl2, TEA, DME, 100 °C, 1 h, μW, 54%; (h) K2CO3, MeOH, rt, 1 h, 72%; (i) vinyl boronic acid MIDA ester, Pd(PPh3)4, Na2CO3, DME, H2O, 120 °C, 1 h, μW, 21–62%.