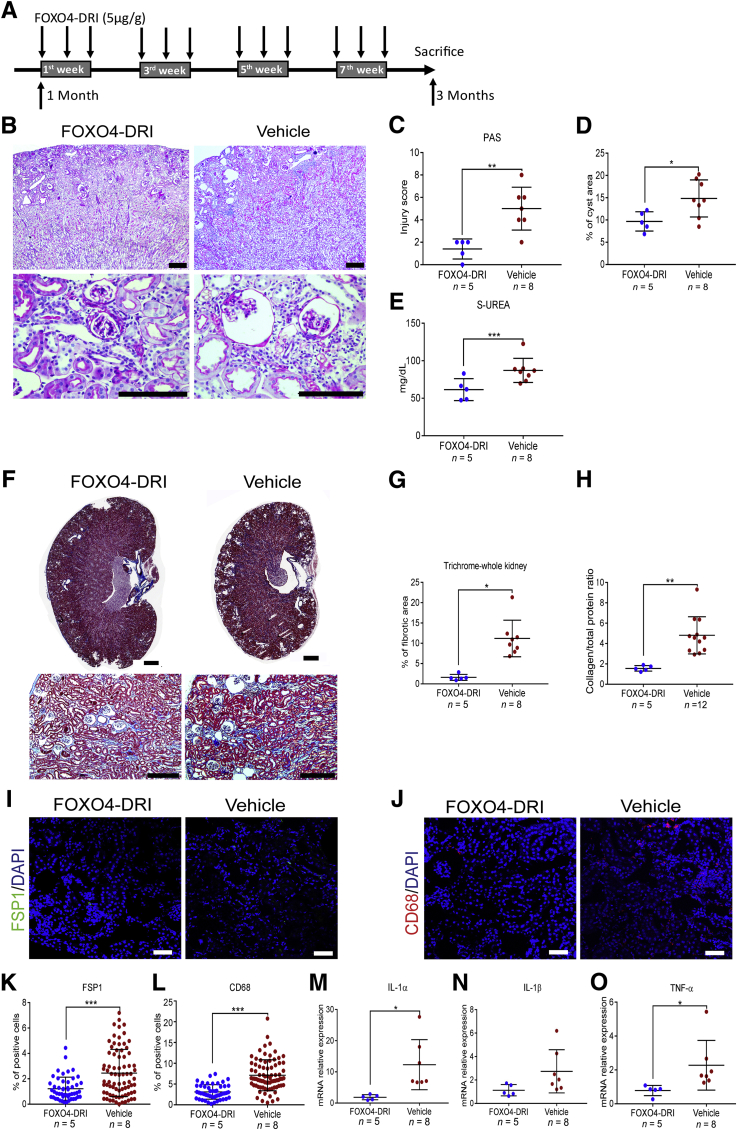
Figure 6.
Treatment with the senolytic agent FOXO4-DRI reduces kidney damage, inflammation, and fibrosis in Glis2 knockout kidneys. A: Schematic representation of the FOXO4-DRI administration protocol used for the Glis2 knockout mice. Starting at 1 month of age, Glis2 knockout mice underwent i.p. injection with the senolytic drug FOXO4-DRI on alternate days, during weeks 1, 3, 5, and 7 after initiation of the experiment, and they were sacrificed at 3 months of age. B: Representative bright-field microscopy images of periodic acid–Schiff (PAS)–stained kidney sections at low (top row) and high (bottom row) magnification for Glis2 knockout mice treated with FOXO4-DRI or vehicle. C–E: Comparison of damage in kidney sections from the two treatment groups, as assessed by PAS staining–based injury score (C), percentage of cystic area, as calculated by digital image analysis (D), and serum urea concentrations (E). F and G: Analysis of fibrosis defects in both treatment groups based on Masson's trichrome staining. F: Representative images of trichrome-stained kidney sections at low (top row) and high (bottom row) magnification, for mice treated as in A. G: Quantification of trichome levels in F by digital image analysis. H: Ratio of collagen/total protein content in kidneys of mice treated as in F. Analysis of inflammation in both treatment groups. I and J: Representative immunofluorescence confocal images of sections of kidneys for the fibroblast marker fibroblast specific protein 1 (FSP1) (I) and the macrophage marker CD68 (J). K and L: Quantification of marker expression in I and J, respectively, by digital image analysis. M–O: Analysis of inflammation in both treatment groups, at the RNA expression level. P values were calculated by two-tailed t-test. Numbers of mice per group are reported in the panels. Data are expressed as means ± SD (C–E, G, H, and K–O). *P < 0.05, **P < 0.01, and ***P < 0.001. Scale bars: 1 mm (B and F, top row); 200 μm (B and F, bottom row); 20 μm (I and J). TNF-α, tumor necrosis factor-α.