Figure 4.
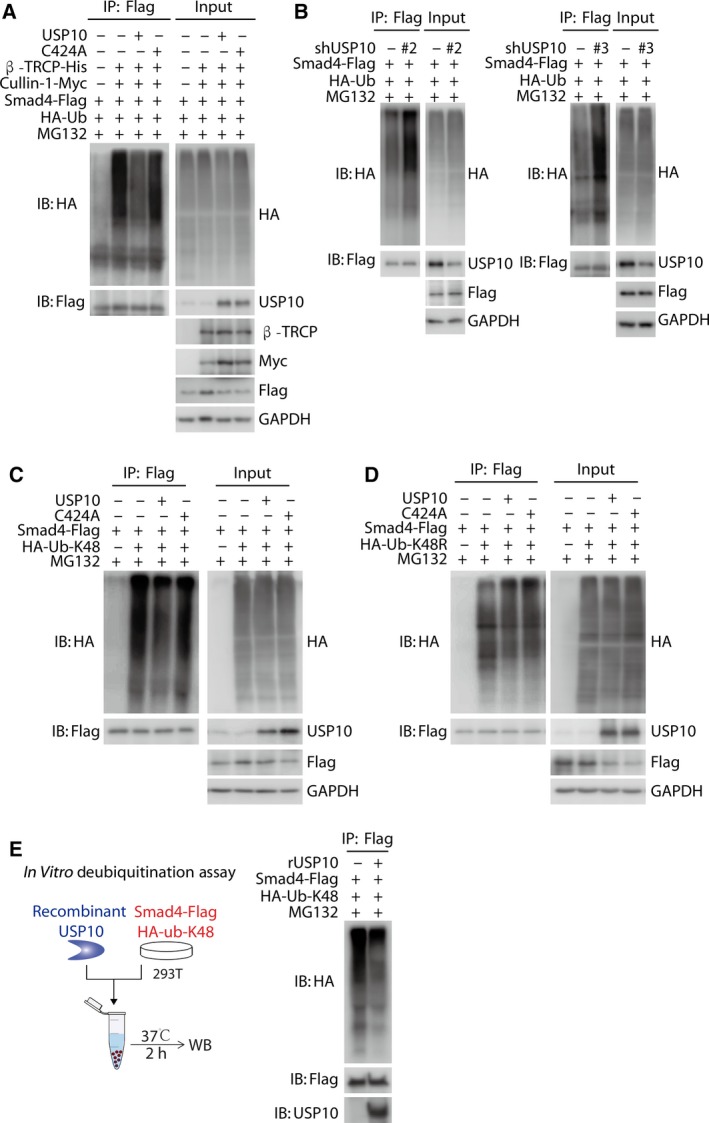
USP10 deubiquitinates and stabilizes Smad4 through the removal of Lys‐48‐linked ubiquitin chains. (A) Co‐expression of USP10‐WT but not USP10‐C424A significantly decreases Smad4 ubiquitination. 293T cells were transfected with expression plasmids encoding for HA‐tagged ubiquitin, Flag‐tagged Smad4, either alone or in combination with His‐tagged β‐TRCP (a known E3 Ligase of Smad4), Myc‐tagged cullin‐1, and nontagged USP10 (WT or C424A). Cells were treated with 10 μm MG132 for 8 h before being harvested. Total cell lysates were immunoprecipitated with an anti‐Flag antibody, and the polyubiquitylated Smad4 protein was detected by the anti‐HA antibody. (B) Depletion of USP10 markedly increased Smad4 ubiquitination. 293T cells infected with lentivirus encoding the indicated shRNAs were transfected with HA‐tagged ubiquitin and Flag‐tagged Smad4 and were treated with 10 μm MG132 for 8 h before being harvested. Total cell lysates were immunoprecipitated with an anti‐Flag antibody, and the polyubiquitylated Smad4 protein was detected by the anti‐HA antibody. (C, D) USP10‐WT significantly decreased Lys‐K48‐linked polyubiquitination on Smad4, but imposed little effect on Lys‐K48R‐linked polyubiquitin chains. HA‐tagged ub‐K48 (Lys‐48 only) or ub‐K48R (Lys‐48R) was cotransfected with empty vectors, Flag‐tagged Smad4 and nontagged USP10 (WT or C424A) into 293T cells, which were treated with 10 μm MG132 for 8 h before being harvested. Total cell lysates were immunoprecipitated with anti‐Flag antibody, and the polyubiquitylated Smad4 protein was detected by the anti‐HA antibody. (E) Bacterial‐expressed recombinant human USP10 (rhUSP10) effectively removed the Lys‐K48‐linked polyubiquitination from Smad4 in vitro. Flag‐tagged Smad4 and HA‐tagged Lys‐K48‐linked ubiquitin mutant were transfected into 293T cells. Subsequently, polyubiquitinated Smad4 from the cell lysate pulled down by anti‐DYKDDDDK (anti‐Flag) IP resin and incubated with rhUSP10 protein for 2 h at 37 °C in vitro. Lysates were immunoblotted with indicated antibodies.
