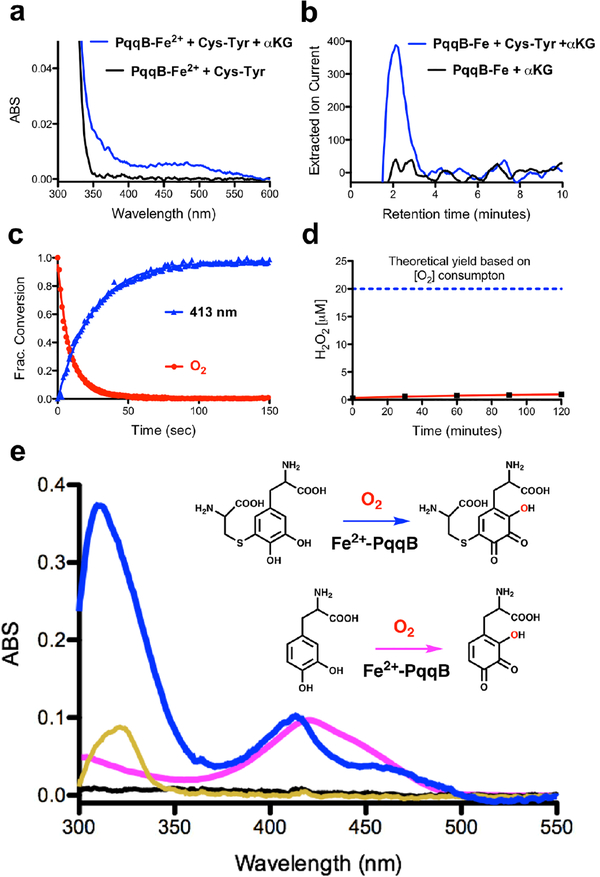
Figure 3. Reaction details of Fe2+-dependent PqqB hydroxylation.
a) and b) Reaction with Cys-Tyr: a) UV-visible spectra of PqqB, Fe2+ and Cys-Tyr in solution (black) and after addition of β-KG (blue). b) LC-HRMS extracted ion chromatograms quantifying the succinate fragment ion by tandem MS (m/z = 73.33, [M-H]−) relative to a succinate standard (Fig. S6). Succinate formation is seen for reaction mixtures of PqqB, Fe2+, α-KG and Cys-Tyr (blue), but not in the control in absence of Cys-Tyr (black). c) and d) Reaction with Cys-DOPA: c) Reaction time course showing O2 consumption (red), and 413-nm absorption band formation (blue). d) Absence of hydrogen peroxide production during Fe2+-PqqB hydroxylation of Cys-DOPA. e) UV-visible spectra of anaerobic PqqB and Cys-DOPA (black). Addition of FeSO4 results in charge transfer (gold). Exposure to O2 leads to rapid formation of OH-Cys-DOPA quinone (blue). An analogous reaction is conducted with L-DOPA to give OH-DOPA quinone (magenta).