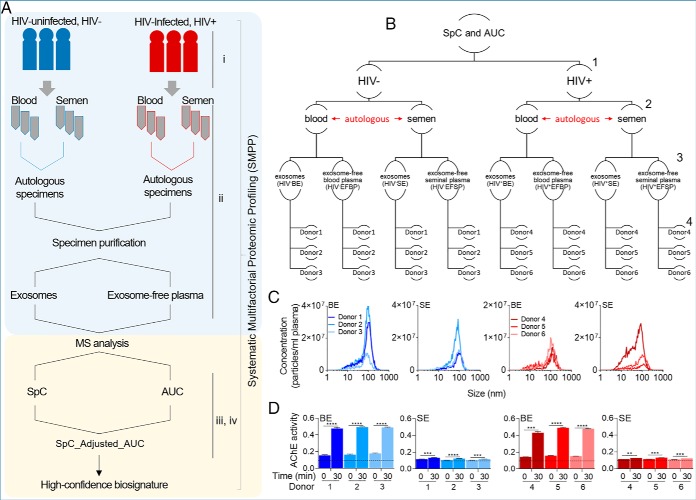
Fig. 1.
Overview of the sample preparation, analysis, and biophysical characterization of body-fluid exosomes. A, Study pipeline and proteomic workflow from participant clinical group to sample collection, and data analysis. B, Hierarchical representation of the studied variables. Numbers to the right represent variable levels, including 1- clinical group (HIV- versus HIV+), 2- body-fluid type (blood versus semen), 3- protein compartmentalization (exosomes versus exosome-free fractions), and 4- donors (3 donors in each group). C, Physical characteristics of BE and SE, showing exosome concentration and size as determined by Nano Tracking Analysis. d, Quantitation of AChE activity of 50 μg BE and SE for each uninfected and HIV infected participant at time 0 and 30 min. Dotted line correspond to the AChE activity of PBS negative control which was unchanged at time 0 and 30 min. Data are reported as the individual mean of triplicate measurements and error bars are S.D. Statistical significance for AChE activity was determined between time 0 min and 30 min for each donor by two-tailed paired Student's t test. **** = <0.0001, *** = <0.001, ** = <0.01, * = <0.05.