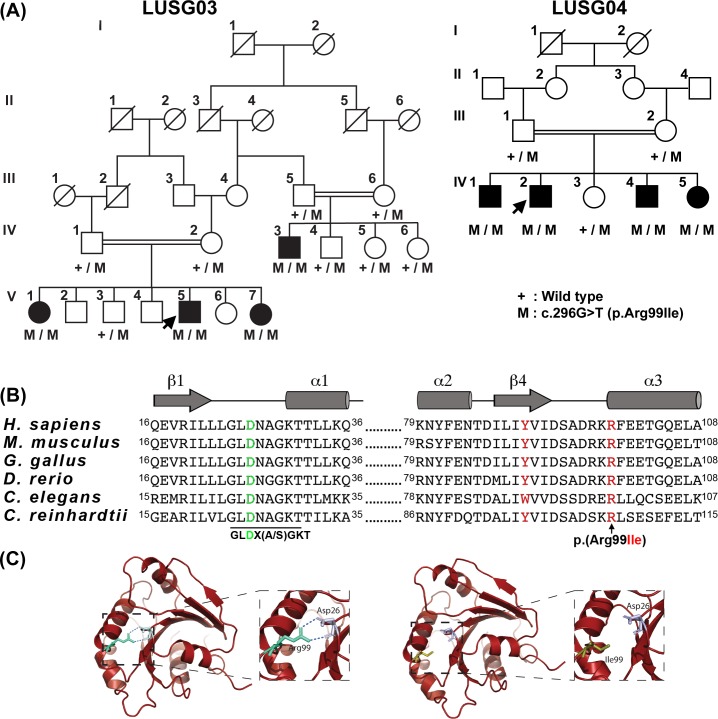
Figure 1.
A missense variant of ARL3 is associated with cone rod dystrophy in two large consanguineous Pakistani families. (A) Segregation of ARL3 allele in families LUSG03 and LUSG04. Filled and empty symbols represent affected and unaffected individuals, respectively. Double lines indicate consanguineous marriages. The genotypes (wild type, heterozygous, and homozygous mutant) of the ARL3 mutant allele are also shown for each of the participating families members. Arrows indicate probands. (B) Human ARL3 has six exons. The c.296G>T variant is located in exon 5. The ARL3 protein has a GTP-binding domain, which harbors the p.(Arg99Ile) variant. ClustalW multiple amino acid sequence alignment of ARL3 orthologs shows that p.Arg99 (red) is highly conserved across species. Shown also is the p.Tyr90 variant (red), known to be mutated in two families with dominantly inherited retinitis pigmentosa. The positively charged guanidino side chain of Arg99 is required to form ionic bonds to stabilize the orientation of the negatively charged side chain of Asp26 (green), which is central to the canonical G-1 motif (aka P-loop) found in GTP-binding proteins, conserved in the ARF family in the sequence GLD26X(A/S)GKT. (C) A homology model of the GTP-binding domain of ARL3. Replacement of arginine with isoleucine at residue 99 variant may alter/reduce the affinity of the protein for guanine nucleotides, resulting in an apoprotein that are much less stable in solution.