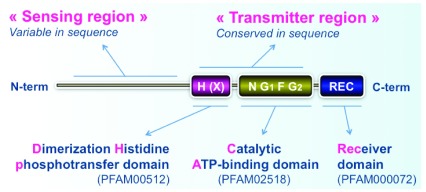
Figure 2. Canonical structure of histidine kinases (HKs).
HKs are composed of a set of variable and conserved domains. The first region corresponds to a highly variable, typically N-terminal sequence that determines which stimulus is perceived by the HK. This region is referred to as the “sensing domain”. The central “transmitter region” is composed of two conserved domains: a dimerization histidine phosphotransfer (DHp) domain (His kinase A, HisKA; Pfam ID PF00512, or other subfamily such as HisKA_2, HisKa_3) and a catalytic ATP-binding (CA) domain (histidine kinase-like ATPase catalytic, HATPase_c; Pfam ID PF02518). The DHp domain includes an H-box, usually containing the phosphorylatable histidine, and an X-box. The CA subdomain includes four distinct sequence motifs: the N-, G1-, F-, and G2-boxes. In contrast to prokaryotic HKs, most eukaryotic HKs contain an additional C-terminal RR receiver (REC) domain (Response_reg; Pfam ID PF00072) that includes a phosphorylatable aspartate residue. Thus, eukaryotic HKs are generally called “hybrid HKs” 26.