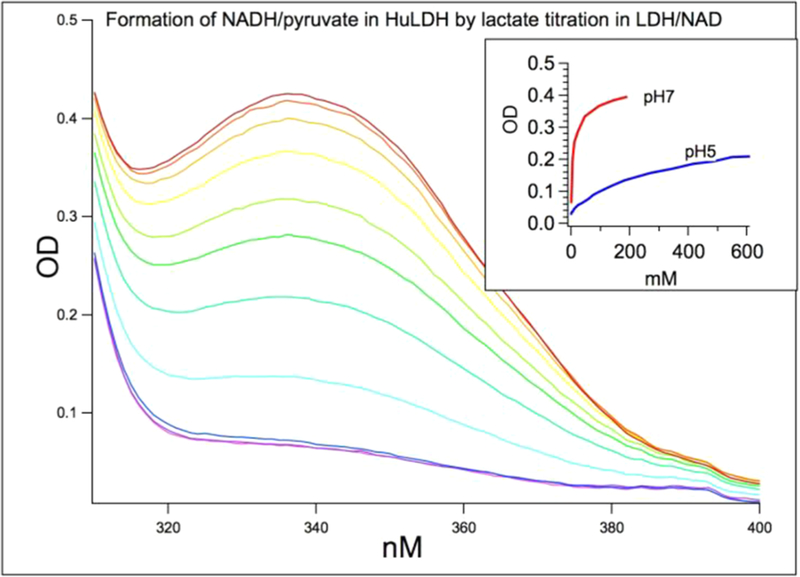
Figure 2.
By lactate titration with hhLDH and NAD at pH 7 and pH 5, using NADH absorbance at 335 nm as the monitor for hhLDH· NADH·pyruvate ternary complex formation. Shown is the lactate titration at pH 7 from 5 to 191 mM. About 35–40% of bound NAD is in the NADH form at pH 7 when 80 mM lactate is reached. Only 10–15% are in the NADH form at pH 5. Thus, we are only able to study pyruvate C2=O bond polarization in the pyruvate side of the Michaelis complex by IR at pH 7. The initial concentrations of hhLDH and NAD+ were 7 mM each. High enzyme and cofactor concentrations ensure that NADH species are bound within the ternary complex.