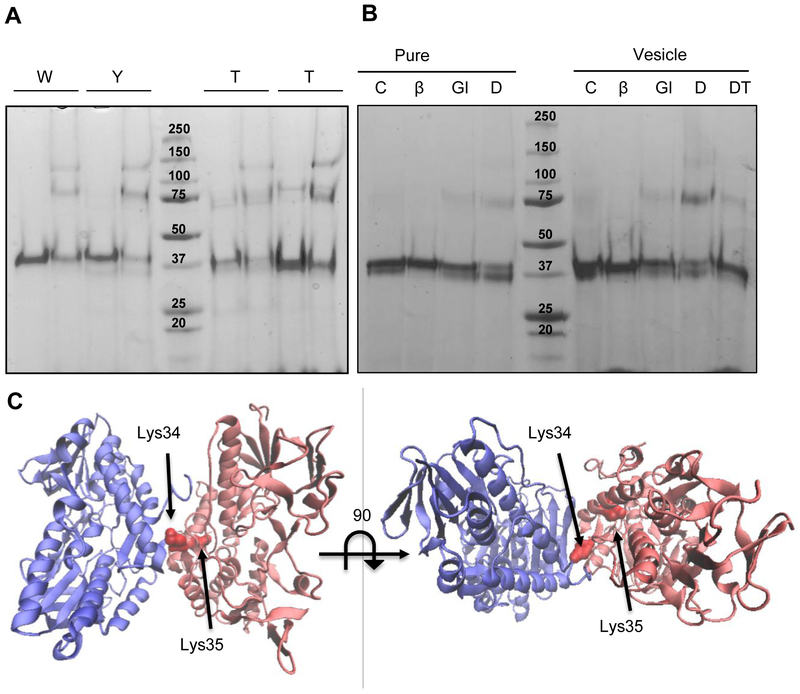
Figure 2. Oligomeric state of WT and soluble mutants by crosslinking assay.
(A) 4 μM (0.2 mg/ml) of WT and mutated protein was run in an SDS-PAGE after a 5 min incubation in the absence (−) or presence (+) of 1.25 mM glutaraldehyde. (B) 4 μM (0.2 mg/ml) of WT protein were incubated in the absence (C: control) or presence of different crosslinkers (Glu: glutaraldehyde; DT: DTSSP) and β-mercaptoethanol (DTβ: DTSSP+ β-mercaptoethanol), without or with lipid vesicles as indicated. Controls were run without and with β-mercaptoethanol (βm). (C) C. maquilingensis SQR model (red) overlapped with the structure of the A. ferrooxidans SQR dimer (blue) indicating the putative lysines involved in DTSSP crosslinking.