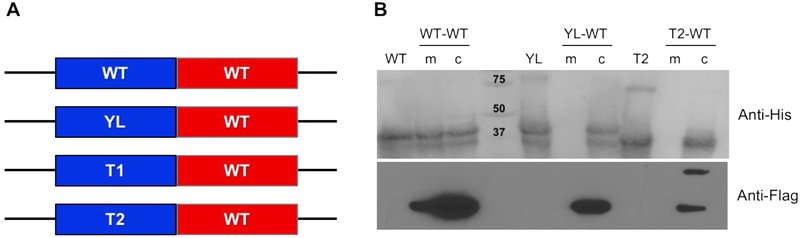
Figure 3. Oligomeric state of WT and soluble mutants by double-tag protein approach.
(A) Constructs containing His-tagged (blue) and Flag-tagged (red) SQR homo- and heterodimers. (B) Ni-NTA purified samples were run in SDS-PAGE, transferred to PVDF membranes and developed by anti-His-tag and anti-Flag-tag antibodies. WT, YL and T2 lanes correspond to His-tagged homodimers (synthesized using single-copy constructs). WT-WT, YL-WT and T2-WT lanes correspond to His-tagged and FLAG-tagged homo- and heterodimers, purified from membrane (m) or cytosolic (c) fractions.