Figure 1: Number of studies by study type and species in ToxRefDB v2.
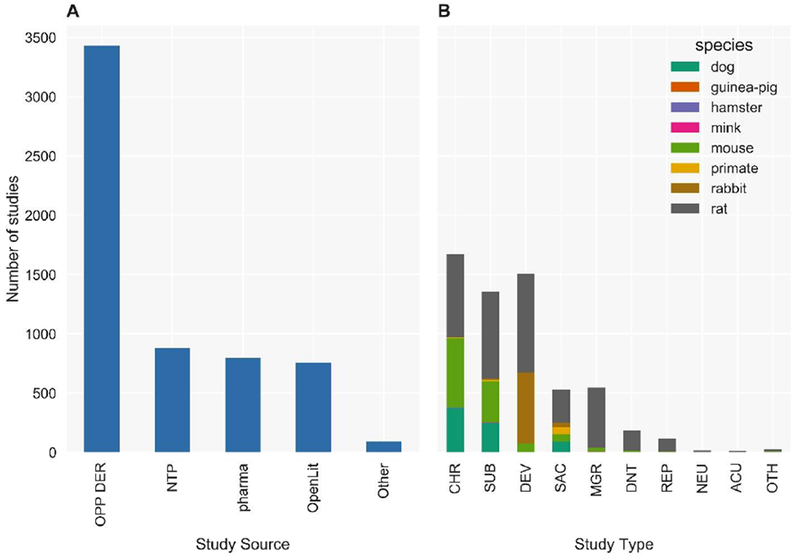
(A) ToxRefDB contains over 5,900 animal toxicity studies from a variety of sources include Office of Pesticides Programs Data Evaluation Records (OPP DER), National Toxicology Program study reports (NTP), pharmaceutical preclinical testing (pharma), open literature (OpenLit), and others (Other). (B) The study designs include chronic (CHR), sub-chronic (SUB), developmental (DEV), subacute (SAC), multigeneration reproductive (MGR), developmental neurotoxicity (DNT), reproductive (REP), neurotoxicity (NEU), acute (ACU), and other (OTH) for numerous species, but mostly for rat, mouse, rabbit, and dog.
