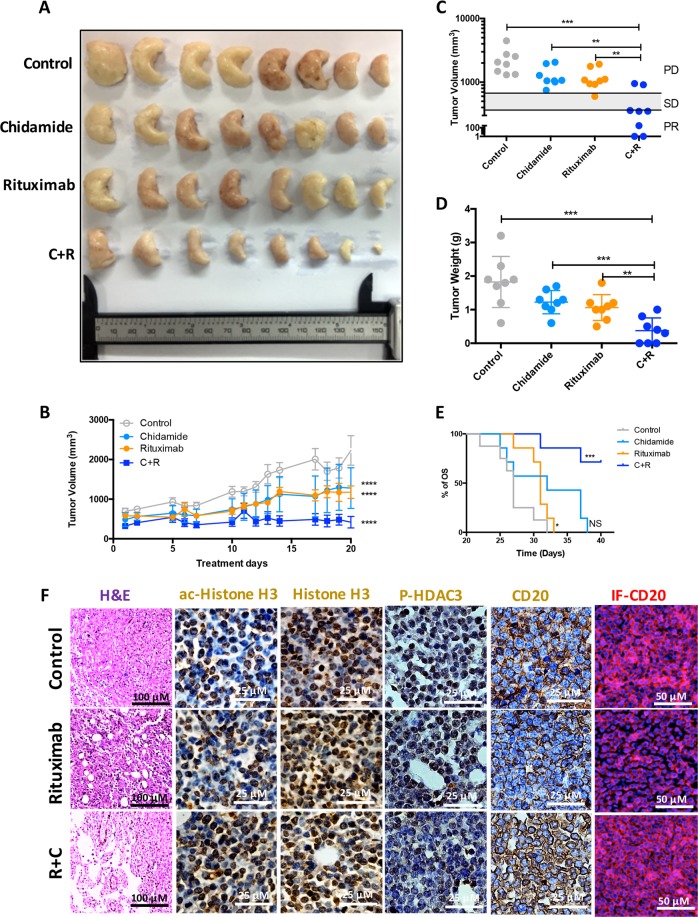
Fig. 7. Effect of Chidamide on Rituximab-induced tumour growth inhibition xenograft mice.
a A collection of DLBCL tumours from human DLBCL tumour-bearing xenograft mice after the endpoints of each indicated treatments (n = 32). b, c Comparison of tumour volumes. Human DLBCL (OCI-Ly7)-bearing BALB/c nude mice were treated with Chidamide and Rituximab according to dosing schedules. Tumour volume was measured, calculated daily and presented as mean volume ± SD. Comparison between final and initial tumour volumes was used to evaluate treatment responses. CR complete response, PR partial response, SD stable disease, and PD progressive disease. d Comparison of tumour weights. Data shown are mean ± SD. e Kaplan Meier overall survival (OS) curves of tumour-bearing xenograft mice. Four subgroups, vehicle control treatment (n = 8), Chidamide treatment (n = 8), Rituximab treatment (n = 8) and Chidamide + Rituximab treatment (n = 8) from two independent experiments. C + R means combined treatment with both Chidamide and Rituximab. f Counteracting effect of Chidamide on Rituximab-induced down-regulation of CD20. Tumour samples were fixed and sliced and stained with H&E (the left column); histochemical chemistry staining of ac-Histone H3, Histone H3, P-HDAC3 and CD20 (the middle columns); immunofluorescent staining of CD20 (the right column). Images were taken with a Nikon microscope (original magnification ×100, ×200 and ×400). Data presented were from three independent experiments.