Abstract
Convulsive status epilepticus is a relatively common emergency condition affecting individuals of all ages. The primary goal of treatment is prompt termination of seizures. Where first line treatment with benzodiazepine has failed to achieve this, a condition known as established status epilepticus, there is uncertainty about which agent to use next. The Established Status Epilepticus Treatment Trial is a 3 arm (valproate, fosphenytoin, levetiracetam), phase III, double blind randomised comparative effectiveness study in patients aged 2 years and above with established convulsive status epilepticus. Enrollment was completed in January 2019, and the results are expected later this year. We discuss lessons learnt during the conduct of the study in relation to: ethical considerations; trial design and practical implementation in emergency settings; including paediatric and adult populations; quality assurance and outcome determination where treating emergency clinicians may lack specialist expertise. We consider ESETT is already informing both clinical practice and future trial design.
Keywords: Status epilepticus, methodology, clinical trial, valproate, levetiracetam, phenytoin
1. Introduction:
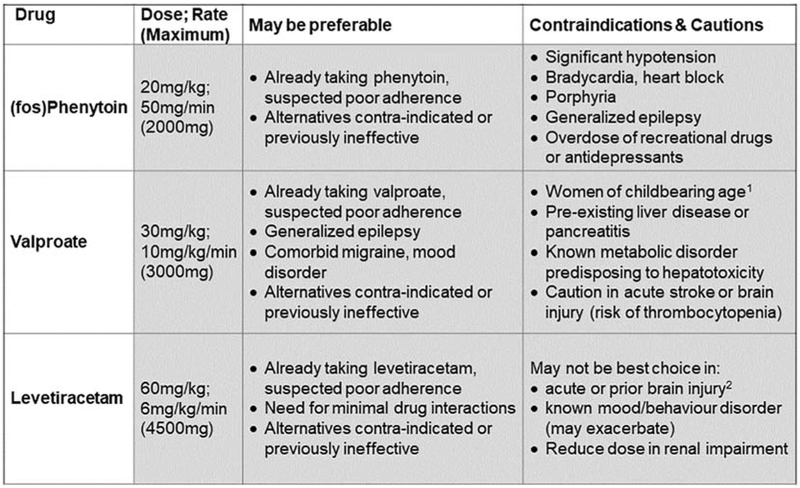
Convulsive Status Epilepticus (SE), defined as convulsive seizures continuing, or recurring without recovery in between lasting more than 5 minutes [1], is a relatively common emergency condition, affecting individuals of all ages. The primary goal of treatment is to terminate seizures as promptly as possible, as the risk of adverse consequences increase with increasing seizure duration, particularly in relation to the first few hours [2,3]. Such complications include neurological sequelae, systemic complications and death. Good evidence now supports initial treatment with benzodiazepines, as reflected in all current guidelines [4]. However, up to a third of convulsive status epilepticus patients continue to have seizures despite adequate doses of benzodiazepines; these patients are considered to have established status epilepticus (ESE). Alternatives to the mainstay of 2nd line treatment thus far, (fos)phenytoin, have gained in popularity in recent years, with data from case series and open studies supporting clinical equipoise [5] between valproate [6], (fos)phenytoin and levetiracetam [7]. Each has its own potential advantages and disadvantages, as summarized in Figure 1. However, there had been no controlled, randomized, blinded clinical trials to compare the efficacy and tolerability of currently available treatments for ESE. A three arm study was the maximum felt feasible logistically, though other agents were considered. Phenobarbitone has proven efficacy in SE [8] but is often not well tolerated as maintenance therapy [9,10], so is rarely used in clinical practice unless other agents have failed. Lacosamide was emerging as a drug of potential interest [11], but with few prospective studies. The safety of rapid intravenous administration in children had also not been established [12]. This informed the final drug selection.
Figure 1. Effective treatment options for established status epilepticus.
(fos)Phenytoin doses shown are for phenytoin, or phenytoin equivalents for fosphenytoin. 1Relative contraindication. Status epilepticus also poses a risk to the woman, and her unborn child. In an emergency situation, especially in a generalized epilepsy or where Levetiracetam is contraindicated, seizure control should take priority. 2 Relative contraindication. This patient group anyway at high risk of fatigue and mood disorders, so may be more vulnerable to these adverse effects on levetiracetam.
The Established Status Epilepticus Treatment Trial (ESETT) had its origins at the 2nd London-Innsbruck Status Epilepticus Colloquium, now 10 years ago [13]. Although originally planned as an international study, the use of a novel Bayesian adaptive randomization design [14] to improve efficiency, and minimize the number of patients randomized to a potentially inferior treatment, enabled recruitment to take place in the United States alone. After enrollment for 27 months, ESETT completed enrollment in January 2019, having met a pre-defined stopping boundary on interim analysis. The results are currently being analysed and prepared for a separate publication, and other secondary papers also expected to follow. In this article we discuss some of the practical and methodological challenges involved, and lessons learnt to inform future trial design in SE.
2. ESETT summary
ESETT was designed to determine the most effective and/or least effective treatment of ESE among patients older than 2 years, comparing fosphenytoin (fPHT), levetiracetam (LVT) and valproate (VPA) in a phase III comparative effectiveness study. The trial methodology was presented at the 2013 Status Epilepticus Colloquium, and published in the proceedings [15]. Briefly, ESETT randomised 478 patients across the 3 treatment arms (Table 1) between November 2015 and December 2018, in a multicentre double-blind study.
Table 1.
ESETT study drug doses, formulation and maximal administration rates
| Study Drug (formulation) | Dose Mg/kg |
Study Maximum | |
|---|---|---|---|
| Rate mg/kg/min | Dose mg | ||
| Fosphenytoin (16.66 mgPE/ml) | 20 | 2 (PE) 1 | 1500 (PE) |
| Valproate (33.33mg/ml) | 40 | 4 2 | 3000 |
| Levetiracetam (50mg/ml) | 60 | 6 | 4500 |
PE = phenytoin equivalents. All drugs were manufactured for the study and stored in pre-randomized study boxes in the emergency department refrigerator, colour coded by the three age groups. All were infused over 10 minutes, at a rate determined by patient estimated or known weight.
Fosphenytoin can be given at 2.2 mgPE/kg/min
valproate at 10mg/kg/min, but the maximum rates were adjusted for the study to maintain the blind. Study boxes included a prompt card, weight/rate table, and an electronic protocol assist device, which alerted the study team that a patient had been randomised and provided timed audible and visible prompts.
Patients were enrolled under Exception from Informed Consent (EFIC) rules across 58 sites, utilizing the Neurology Emergency Treatment Trials network (NETT) and Pediatric Emergency Care and Applied Research Network (PECARN). Patients over the age of 2 years, with witnessed clinically apparent convulsive seizures in the emergency department for at least 5 minutes despite adequate doses (discussed later) of prior benzodiazepines for that episode were included. Randomisation was stratified by three age groups, 2-18, 19-65 and 66 years and older. The primary outcome measure was clinical cessation of seizure activity and improving mental status (Richmond agitation-sedation scale [16]), without serious adverse effects or further intervention at 60minute after initiating administration of the study drug. Any of life-threatening hypotension or cardiac arrhythmia, intubation, or a requirement for additional antiseizure drugs within the first 60 minutes were regarded as a treatment failure on the primary outcome. Other secondary outcomes are listed in Table 2.
Table 2.
Secondary outcomes in ESETT
| Secondary outcomes in ESETT, after study drug initiation (time 0) | |
|---|---|
| Admission to ICU | Mortality |
| Time to seizure termination (minutes) | Acute anaphylaxis within 6 hours |
| Length of stay (ICU and hospital) | Elevated liver transaminases or ammonia |
| Seizure recurrence within 60minutes to 12hours | Purple glove syndrome |
ICU = intensive care unit.
There was an a priori intent to undertake analysis both of the whole cohort, and the age-stratified groups, reflecting known age-associated differences in etiology and outcome [17]. Subjects were followed until hospital discharge, or 30 days from enrollment, which ever was sooner. Interim analyses were undertaken after enrollment of every 100 patients from 300 patients onwards to inform response-adaptive randomization, and to determine early success or futility. Statistical modelling and power calculations were based on one treatment being effective in 65%, against 50% in the other arms, and a >0.975 probability of identifying a most or least effective drug, with 90% power.
3. Key Lessons
3.1. Ethical considerations in life threatening emergency conditions
Clinical trials of life-threatening illnesses pose special challenges to research ethics, study design and implementation. When necessary to conduct clinical trials needed to improve the care and outcomes of critically ill and injured patients, research without consent in the emergency setting is internationally recognized as acceptable practice, within the Declaration of Helsinki [18]. ESETT has been an opportunity to learn more about research without consent in the emergency setting. Patients in generalized SE are unconscious, so an informed consent process is not possible, and patients cannot say whether or not they would want to participate in the research. Furthermore, the alternative process of identifying and obtaining surrogate informed consent from a legally authorized representative (LAR) is not practicable in SE, because the emergency treatment being studied must be initiated as quickly as possible to safely care for the patient. Identification of the optimal emergency care for those in SE in ESETT therefore required use of exception from informed consent (EFIC) for emergency research, which in the United States, is detailed in the Code of Federal Regulations (21 CFR 50.24) [19]. Because informed consent is not feasible, additional safeguards are required, including community consultation, public disclosure, and notification of the LAR as soon as feasible. For FDA research, the FDA must also approve the EFIC. The UK and other European nations have similar exceptions for emergency research, but the requirements to obtain approval for such research are not fully harmonized [20]. This contributed to the decision to obtain funding and approval for ESETT only in the United States.
Because EFIC studies enroll patients without prior consent, it is ethically imperative to make the studies as efficient as possible and to minimize patient exposure to potentially ineffective or unsafe therapies. ESETT was designed with this in mind. Using Bayesian adaptive randomization and a primary Bayesian analysis, we were able to 1) potentially reduce the overall sample size; 2) minimize exposure to the medications likely to be the least effective; and 3) perform more frequent interim analyses without the large statistical penalties incurred by frequentist statistical analysis.
Subjects in ESETT, or their LAR, were notified of the study enrollment and approached for consent to continue participation in the study as soon as practicable, most often within a hour or so after enrollment. Relatively little is known about the experience of those notified of EFIC enrollments and then asked about consent-to-continue. We embedded an empirical ethics survey study within ESETT to learn more about the normative responses to consent after EFIC enrollments. The results revealed very high levels of acceptance of the specific enrollment, and high levels of acceptance of EFIC in general [21].
3.2. Trial design and practical implementation in emergency settings
Conducting research in the emergency setting also requires study design and implementation strategies that allow the investigation to integrate efficiently and relatively seamlessly into the acute medical care of the patient/subject. Integration needs to be planned from the very beginning and involves design consultation with clinical medical and nursing providers and site study coordinators. Examples of design features in ESETT included to allow efficient implementation includes step-forward randomization, in which a “use-next” study kit (Figure 2) is pre-randomized and placed in the pharmacy dispensing system to avoid delay in administration of study drug during treatment of SE [22]. Other design features intended to simplify implementation include the use of measuring tape like length-based-dose-finding tool to determine the volume of study drug to give to children of unknown weight. Like the Broselow tape familiar to emergency providers for pediatric resuscitations, this is a colour coded tool that can be laid down next to a seizing child to immediately indicate the proper dose of study drug based on the child’s length/height. Even small details in study drug manufacturing can make integration into practice easier and more efficient. The ESETT study drug vials were deliberately designed with stoppers that could be directly spiked with an IV drip set, and a vial label that integrated a hanger, so that study drug could be administered straight from the hanging inverted vial without having to be separately drawn up and placed in an IV bag prior to treatment. ESETT also created an iOS-based protocol-assist-device (Figure 2 and 3) that performed several functions that made bedside enrollments easier. These ranged from activating study teams, reminding clinical teams of eligibility criteria and protocol steps, collecting time data from voice annotations, and facilitating unblinding after determination of the primary outcome if deemed necessary for patient care.
Figure 2.
A. Open “use next” study kit.
From top to bottom, left to right: dose administration chart, instruction card, the protocol assist device, charging cable and plug, coiled colour coded length estimation tape, space for study medication. The dosing chart included guidance based on estimated weight (top of chart as shown) or length (colour coded, bottom)
B. Colour coded “use next study boxes”.
Coded by age stratification groups (2-17years; 18 – 65 years; 66 years and older)
Figure 3. Protocol assist device screenshots.
Illustrative screenshots from a protocol assist device. Top row left to right: Start screen; Confirmation eligibility followed by protocol start; audible and visual prompts for the clinical team reminding them verbal comments can be audio-recorded throughout; prompt to stop infusion at 10 minutes. Bottom row left to right: prompts for assessment seizure control (2 questions at each time point) at 20miutes, and 60 minutes; submission results; facility for emergency unblinding if required.
ESETT involved 58 sites, requiring a substantial investment in training local staff from the outset. As enrollment rates differed substantially between sites, with sometimes months between enrollments, the need for close monitoring and responding to protocol deviations, and frequent re-training also became apparent. A range of tools including on-line resources, investigator meetings and individual site/investigator feedback were used throughout. Simulation training was identified as a best practice in this study and is being adopted widely for other studies in the Pediatric Emergency Care Applied Research Network (PECARN).
3.3. Pharmacological issues
From a pharmacology perspective, elements of the ESETT design triggered a number of lessons learned. These elements included: 1) a blinded study 2) a comparative efficacy trial with 3 commercially-available drugs, and 3) enrollment of subjects as young as 2 years and above.
In order to maintain the blind, the 3 drugs, fosphenytoin (FOS), levetiracetam (LEV), and valproic acid (VPA), had to have the same appearance and be administered in the same volume and infusion rate despite the fact that the drugs had different mg/kg doses. Thus, the formulations for the drugs had to be modified so that for any given subject, the same volume and infusion rate would be administered regardless of the medication to which the subject had been randomized. The infusion rates were dictated by the FOS product label which stipulates a maximum infusion rate because of the known risk of hypotension (Table 1). One possible implication of infusion rate standardization is an impact on the true potential efficacy of LEV and VPA. Further, ESETT utilized weight-based dosing; but the dose was capped at 75 kg because of either the product label or relatively sparse dosing information in the literature. Given that approximately one third of the ESETT subjects weighed greater than 75 kg, a significant number of subjects could have received lower than the mg/kg calculation would have advised. This may be especially important because patients weighing more than 75 kg are likely to have more body fat and therefore higher volumes of distribution for the generally lipophilic medications used to treat ESE. Lastly, because of the dosing scheme and need for blinding, the fill volume was 100 mL to accommodate the highest adult dose i.e. 90 mls. However, medication dosing is more complicated in children because of the wide range of weights in this age group. Medication errors in the emergency setting are three times as likely in children as adults [23]. Furthermore, given the wide weight range in children larger dosing errors are likely. Despite the inclusion of weight-based dosing cards in the study drug boxes, we experienced two potentially serious medication errors, both in young children, when the entire vial was administered to patients rather than a weight-appropriate dose. The use of pre-filled syringes and/or category-based dosing (e.g. one adult dose and one child dose) were best practices used in the RAMPART prehospital SE study [24] but were not feasible for ESETT, and if attempted would also have added significantly to manufacturing costs and logistical challenges. For future studies involving children in emergency situations, it may be prudent to limit enrollment to sites with dedicated pediatric emergency departments, in which staff are accustomed to weight-based dosing. In general, such sites have higher volumes of pediatric patients and teams may be more facile with critically ill pediatric patients. While this may limit generalizability, it will provide additional safety protections which are key in a study such as this.
Because the drugs tested in ESETT were commercially available, the investigators had the choice between using the commercial drug product or the active pharmaceutical ingredient (API) as the starting point for manufacturing the investigational formulations. The commercial products are generally available, and quality is assured. However, purchase of commercial products tends to be more expensive and may require prior approval from the drug company for use in a clinical trial. Purchasing API is typically less expensive but can be difficult to source and due diligence is needed to ensure quality as evidence by a certificate of analysis (CofA) and a track record of manufacturing API for products used in humans. We found that relying on the CofA is not always adequate. Further, use of API may result in need for additional regulatory documentation as the IND must include the manufacturing processes. Even though API may be inexpensive, the additional work required to manufacture it into the desired formulation may make it less cost effective. Investigators also need to take into consideration the expiration of the raw materials (including drug container supplies) so as to avoid early expiration of materials prior to manufacturing or the end of study. Prior to initiating any aspect of drug manufacturing, testing requirements and assays used for quality and stability testing need to be determined, along with their associated costs. Unexpected test results can happen, adding additional time and costs. We learned that there needs to be a standard operating procedure (SOP), written in advance of initiating manufacturing, that addresses what to do when quality or stability results fail to meet specifications. In ESETT, blinding precluded the inclusion of expiration dating on vials. Further, expiration dates were extended through the course of the study using commonly recognized expiration forecasting methods. The expiration date for each drug was maintained in the study database. In some cases, the investigational pharmacies at the clinical sites required additional information to satisfy local regulations. Information about and access to expiration dating needs to be presented in a manual of procedures and emphasized in training. Lastly, temperature excursion occurred during shipment and storage at sites. As those arose, we created an SOP to handle such issues. Again, this would ideally be done in advance of initiation of the trial.
In relation to a pharmacokinetic sub-study added after ESETT commencement, we also found that obtaining PK samples within ESETT was challenging. Blood collection for PK required a second line or multiple venepunctures and two blood samples collected in the first 2 hrs of the study. Regardless of EFIC, some institutions determined that blood sampling in children required consent. Most sites followed a procedure where after 60 minutes blood could be collected without consent if child was unconscious or had a second line. If neither of these conditions were met, then consent was obtained prior to blood collection. The use of a device (e.g. PIVO™) that allows one to collect blood from the same line as is used for drug infusion would be a great advance. We also found that it was important to make PK sampling and sample processing procedures simple and integrate blood sampling into the other ESETT procedures. For example, this study utilized population PK modeling and a sparse blood sampling strategy. This data analysis approach allowed for blood sampling windows instead of requiring discreet time points, which provided sites more flexibility in the timing of blood collection.
3.4. Special considerations for children
The ESETT study included both adults and children 2 years of age and older. In addition to the pharmacological and medication dosing issues discussed above, there are two specific considerations relative to the pediatric population. First, while SE is a common occurrence, response rates to initial therapy are high. Therefore the relative infrequency of ESE and eligibility for enrollment in the trial is relatively low compared to trials in new onset SE. Even at large, tertiary pediatric hospitals, as in some adult centres, a subject might be enrolled only once every few months. Again, regular staff training is critical as discussed earlier.
Second, the study excluded children under 2 years of age. This was done for several reasons. One was that the weights of younger children would add additional complexity to the study procedures and increase the potential for dosing errors. Additionally, in the second year of life, febrile SE accounts for approximately three quarters of SE, which may have a different pathophysiology and drug responsiveness profile than other etiologies [25]. Finally, safety concerns about valproate are highest in children under 2 years of age, and pharmacokinetic differences exist between very young infants and other age groups with respect to the study drugs. The first 2 years of life have the highest incidence of status in children so eliminating them was done only after much thought but felt to be necessary for the reasons noted.
3.5. Adjudication and review of data entry
The correct diagnosis and classification of seizure disorders and SE can be challenging, particularly to non-epilepsy specialists. Furthermore, the definitions and classification of both have changed in recent years [1,26,27], reflecting new knowledge and understanding. Even where a prior diagnosis of epilepsy is firmly established, determining whether an episode of SE should be considered unprovoked (i.e. a manifestation of the epilepsy), or provoked (such as by a febrile illness, antiepileptic drug withdrawal/non-adherence, acute toxic, metabolic or other brain events) also requires subspecialist expertise. Given that etiology is a key independent determinant of outcome in SE in general and ESE in particular, whilst the primary outcomes were determined by the treating teams, a central clinical phenomenology core (SS, DL, HRC, NT) was established to determine diagnosis, seizure onset and stop times where possible, and independently also report on the primary outcomes by review of anonymized records, with any information that might disclose study treatment redacted. Initially 20 records were independently scored by all 4 members of the core so we could all agree on uniform criteria for etiology, what constituted provoked SE and other key aspects. Subsequently, all participant records were independently reviewed by at least two of the phenomenology core. DL reviewed all adults, SS all children, and HRC/NT alternating patients irrespective of age. Each independently completed a structured online form, and when both reviews were complete, a web-based iterative process generated a reconciliation report. Where there were discrepancies, consensus was almost always reached between core members on further review, with the option for a third independent review if required using the same process.
Whilst the impact of this effort on the study conclusions is under analysis, anecdotally all members of the core felt this aspect of the study methodology proved to be an essential part of quality assurance. As expected, a number of randomised patients turned out to have diagnoses other than ESE, most commonly psychogenic non-epileptic seizure. Another common occurrence was patients considered by the site to have prior diagnosis of epilepsy, on the basis of previous seizures, but in whom no such diagnosis had been made or was appropriate prior to ESETT enrollment (e.g. recurrent acute symptomatic seizures, often febrile, or only a single prior seizure). Treating teams also more readily attributed episodes to acute provokers, particularly antiepileptic drug withdrawal, even where this had occurred sometimes weeks before or had not occurred, or when the ESE episode did not meet current definitions for acute symptomatic seizures [28]. In these cases, the ESE episode was usually considered by the core to be a manifestation of the subject’s incompletely controlled epilepsy, without specific cause at the time. In those with epilepsy, there was mostly agreement on type and etiology, though in this domain, the importance of ensuring age-appropriate expertise within each pair proved essential. Inevitably the adult neurologists (HRC, DL, NF) were less familiar and experienced in classifying some of the rarer paediatric developmental and epileptic encephalopathies correctly, and the paediatric neurologist (SS) would have been potentially less comfortable with scenarios more commonly encountered in adult, especially elderly, populations.
Independent clinical review of all the source documentation also highlighted considerable variation in the level of detail recorded contemporaneously. Where pre-hospital emergency services had been involved, exact times for the initial call, arrival at the patient, and pre-hospital interventions were almost always clear. For those brought in by other routes, or where individuals had been found already seizing or unconscious, such detail was often vague or missing completely, making estimations of seizure onset sometimes difficult. Once in the emergency department, the 20 minute and 60 minute observations, mandated by the protocol, were usually clear, as were timings of any drug administration. However, determining when clinical seizures terminated, or recurred, were sometimes challenging. Again, two independent reviewers proved useful, as one would often have spotted a small detail the other may have missed.
4. Conclusions
Whilst analysis of the primary and secondary outcomes is on-going, and results eagerly awaited, there is much that has already been learnt from the ESETT study, informing both clinical practice and future trial design.
The successful completion of the ESETT study adds to the body of evidence supporting the ability of well-structured and adequately funded research networks such as NETT and PECARN to deliver high quality studies in emergency conditions. Regardless of the outcome, the recruitment of over 450 patients without any safety concerns at the recommended doses, also substantially adds to the body of evidence supporting the use of levetiracetam and valproate as well as (fos)Phenytoin in convulsive SE. Some guidelines were already starting to include all three agents as options [4]. Institutions and authorities can now have more confidence in doing so, at the doses used in this study, as will for example be reflected in one upcoming highly used resource [29]. ESETT has also demonstrated the utility of the Bayesian adaptive randomization approach in practice. This has potentially reduced the final costs of the study, reduced the risk of exposure to a less effective medication, and prevented on-going randomization when the likely outcome is already known, without the large statistical penalties incurred by frequentist statistical analysis. That the study recruited ahead of target, as was also the case with RAMPART, justifies the careful consideration and investment in key design and implementation features from the outset, such as the pre-randomized colour coded use next kits, protocol assist devices and tools to guide dose.
Recruiting both adults and children, aged 2 and upwards in to the 90s whilst important to inform practice for the broad population at risk, also brought its own challenges. Maintaining the blind with weight based dosing across such a large range was achieved, but with 2 potentially serious dosing errors in children. In future, limiting paediatric recruitment to sites with dedicated paediatric departments may be appropriate. Blinding the study also required us to manufacture the investigational formulations, itself requiring detailed analysis and testing for quality and stability. The costs of this, and of a defined process for responding to and investigating any unexpected test results also needs to be considered from the outset.
ESETT has demonstrated both the feasibility using a web-based platform, and the importance of use of a central core of experts to determine key outcomes in areas where specialist expertise is known to be important, such as in the diagnosis and classification of seizure disorders. The process also highlighted considerable variability in pre-hospital emergency services access to benzodiazepines between sites, and in the quality of documentation both in and out of hospital. This was to some extent anticipated, and one factor that supported the use of the protocol assist device and trial requirement for specific assessments at 20 and 60 minutes.
In summary, ESETT has successfully enrolled both adults and children in a randomized, blinded comparative effectiveness trial in the emergency management of convulsive SE. Because of the study complexity, the implementation and completion of the trial required a multidisciplinary team, including neurologists, emergency physicians, pharmacologists, statisticians, research coordinators, and computer programmers. We present several important lessons for future research in the emergency setting.
LESSONS FROM ESETT – Highlights.
Careful design from the outset is essential for successful delivery of emergency medicine trials
Bayesian adaptive randomization and analysis can support the ethical imperative to optimise study efficiency and safety in Exception from informed consent trials
Design features support practical implementation in an emergency setting included step-forward randomization with “use next” study kits and a protocol assist device
Formulating drugs to maintain blinding can necessitate sub-optimal dosing in some patients, as well as having significant cost implications
Expert independent review of source documentation may be an essential part of quality assurance where specialist expertise is important for diagnosis or classification.
Acknowledgments
Funding: Research discussed in this publication was supported by National Institutes of Health, National Institutes of Neurological Disorders and Stroke under Awards U01NS073476, U01NS088034, U01NS088023, U01NS056975, U01NS059041, and R01NS099653.
Declaration of interests: All authors were supported by the ESETT study grant from NIH/NINDS (U01NS088034). Dr. Chamberlain and the enrolling sites from the Pediatric Emergency Care Applied Research Network (PECARN) were supported by grants from the Health Resources and Services Administration (HRSA), Maternal and Child Health Bureau (MCHB), Emergency Medical Services for Children (EMSC) Network Development Demonstration Program under cooperative agreements U03MC00008, U03MC00001, U03MC00003, U03MC00006, U03MC00007, U03MC22684, and U03MC22685. Dr. Coles reports grants from NIH/NINDS, during the conduct of the study; personal fees from Neurelis Pharmaceuticals, grants from Sollievo, outside the submitted work; Dr. Shinnar reports grants from NINDS, during the conduct of the study; personal fees from UCB Pharma, personal fees from Eisai, personal fees from Insys, outside the submitted work; Prof. Cock reports grants from NINDS, during the conduct of the study; personal fees from Sage Pharmaceuticals Ltd, personal fees from Eisai Europe Ltd, personal fees from UCB Pharma Ltd, personal fees from UK Epilepsy Nurse Specialist Association, non-financial support from Special Products Ltd, non-financial support from International League Against Epilepsy, Epilepsy Certification (education) Task Force, non-financial support from European Academy of Neurology, personal fees from Bial and Eisai, outside the submitted work; Dr. Fountain reports grants from NINDS, during the conduct of the study; grants from SK Lifesciences, grants from Neurelis, grants from Takeda, grants from GW Pharma, grants from Biogen, grants from UCB, outside the submitted work. Dr. Cloyd reports a grant from National Institute of Neurological Disorders and Stroke during the conduct of the study; personal fees from Neurelis Pharmaceuticals, grants from Sollievo, outside the submitted work. In addition, Dr. Cloyd has a patent entitled "Intranasal Drug Delivery" which the University of Minnesota has licensed to Sollievo.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus - Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. 2015;56(10):1515–23. [DOI] [PubMed] [Google Scholar]
- [2].Neligan A, Shorvon SD. Prognostic factors, morbidity and mortality in tonic-clonic status epilepticus: A review. Epilepsy Research. 2011;93(1):1–10. [DOI] [PubMed] [Google Scholar]
- [3].Crawshaw AA, Cock HR. Medical management of status epilepticus: emergency room to intensive care unit. Seizure. 2020;In press. [DOI] [PubMed] [Google Scholar]
- [4].Glauser T, Shinnar S, Gloss D, et al. Evidence-Based Guideline: Treatment of Convulsive Status Epilepticus in Children and Adults: Report of the Guideline Committee of the American Epilepsy Society. Epilepsy Currents. 2016;16(1):48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yasiry Z, Shorvon SD. The relative effectiveness of five antiepileptic drugs in treatment of benzodiazepine-resistant convulsive status epilepticus: a meta-analysis of published studies. Seizure. 2014;23(3):167–74. [DOI] [PubMed] [Google Scholar]
- [6].Trinka E, Hoefler J, Zerbs A, Brigo F. Efficacy and Safety of Intravenous Valproate for Status Epilepticus: A Systematic Review. Cns Drugs. 2014;28(7):623–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brophy GM, Bell R, Claassen J, et al. Guidelines for the Evaluation and Management of Status Epilepticus. Neurocritical Care. 2012;17(1):3–23. [DOI] [PubMed] [Google Scholar]
- [8].Treiman DM, Meyers PD, Walton NY, et al. A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. New England Journal of Medicine. 1998;339(12):792–8. [DOI] [PubMed] [Google Scholar]
- [9].Mattson RH, Cramer JA, Collins JF, et al. Comparison of carbamazepine, phenobarbital, phenytoin, and primidone in partial and secondarily generalized tonic-clonic seizures. N. Engl. J. Med 1985;313(3):145–51. [DOI] [PubMed] [Google Scholar]
- [10].Heller AJ, Chesterman P, Elwes RDC, et al. Phenobarbitone, Phenytoin, Carbamazepine, Or Sodium Valproate for Newly-Diagnosed Adult Epilepsy - A Randomized Comparative Monotherapy Trial. Journal Of Neurology Neurosurgery And Psychiatry. 1995;58(1):44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hofler J, Trinka E. Lacosamide as a new treatment option in status epilepticus. Epilepsia. 2013;54(3):393–404. [DOI] [PubMed] [Google Scholar]
- [12].Fountain NB, Krauss G, Isojarvi J, Dilley D, Doty P, Rudd GD. Safety and tolerability of adjunctive lacosamide intravenous loading dose in lacosamide-naive patients with partial-onset seizures. Epilepsia. 2013;54(1):58–65. [DOI] [PubMed] [Google Scholar]
- [13].Cock HR, group obotE. Established Status Epilepticus Treatment Trial (ESETT). Epilepsia. 2011;52:50–2. [DOI] [PubMed] [Google Scholar]
- [14].Connor JT, Elm JJ, Broglio KR, Investigators EA-I. Bayesian adaptive trials offer advantages in comparative effectiveness trials: an example in status epilepticus. Journal of Clinical Epidemiology. 2013;66(8):S130–S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bleck T, Cock H, Chamberlain J, et al. The Established Status Epilepticus Trial 2013. Epilepsia. 2013;54(SI):89–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale - Validity and reliability in adult intensive care unit patients. American Journal of Respiratory and Critical Care Medicine. 2002; 166(10): 1338–44. [DOI] [PubMed] [Google Scholar]
- [17].Rossetti AO, Hurwitz S, Logroscino G, Bromfield EB. Prognosis of status epilepticus: role of aetiology, age, and consciousness impairment at presentation. J Neurol Neurosurg Psychiatry. 2006;77(5):611–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].World Medical Association. WMA Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects. World Medical Association; 2013. [DOI] [PubMed] [Google Scholar]
- [19].US Department of Health and Human Services. Guidance for Institutional Review Boards, Clinical Investigators, and Sponsors. Exception from Informed Consent Requirements for Emergency Research New Hampshire, USA: US Department of Health and Human Services; 2013. https://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM249673.pdf [Google Scholar]
- [20].Rebers S, Aaronson NK, van Leeuwen FE, Schmidt MK. Exceptions to the rule of informed consent for research with an intervention. Bmc Medical Ethics. 2016; 17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dickert Neal W, Biros Michelle H, Harney Deneil K, et al. Abstract 134: Patients’ Perspectives on Enrollment in Research Using the Exception From Informed Consent: An Integrated Survey. Circulation. 2018;138(Suppl_2):A134–A. [Google Scholar]
- [22].Zhao WL, Ciolino J, Palesch Y. Step-forward Randomization in Multicenter Emergency Treatment Clinical Trials. Academic Emergency Medicine. 2010;17(6):659–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Benjamin L, Frush K, Shaw K, et al. Pediatric Medication Safety in the Emergency Department. Pediatrics. 2018;141(3). [DOI] [PubMed] [Google Scholar]
- [24].Silbergleit R, Durkalski V, Lowenstein D, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. New England Journal of Medicine. 2012;366(7):591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shinnar S, Pellock JM, Moshé SL, et al. In Whom Does Status Epilepticus Occur: Age-Related Differences in Children. Epilepsia. 1997;38(8):907–14. [DOI] [PubMed] [Google Scholar]
- [26].Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE Official Report: A practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–82. [DOI] [PubMed] [Google Scholar]
- [27].Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):522–30. [DOI] [PubMed] [Google Scholar]
- [28].Beghi E, Carpio A, Forsgren L, et al. Recommendation for a definition of acute symptomatic seizure. Epilepsia. 2010;51(4):1528–167. [DOI] [PubMed] [Google Scholar]
- [29].Cock HR. Antiepileptic drugs In: Waldmann C, Soni N, Rhodes A, eds. Oxford Desk Reference: Critical Care. 2nd ed. Oxford, UK: Oxford University press; 2019. (In press). [Google Scholar]