Figure EV2. Synthesis of RO7019009.
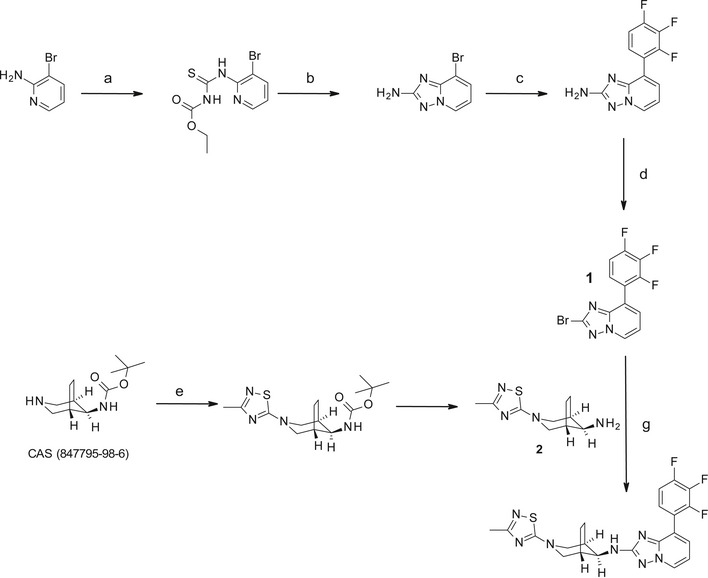
Schematic overview of the steps of the RO7019009 synthesis. Reagents and conditions: a) EtO2CNCS (1.1 equiv), dioxane, room temperature, 3 h, quant.; b) NH2OH.HCl (5 equiv), i‐Pr2NEt (3 equiv), MeOH/EtOH (1/1), room temperature to 60°C, 4 h, 94%; c) 2,3,4‐trifluorophenylboronic acid (1.3 equiv), Cs2CO3 (2 equiv), PdCl2(DPPF). CH2Cl2 (0.1 equiv), dioxane/H2O (10/1), 100°C, overnight, 82%; d) tert‐butyl nitrite (1.5 equiv), Cu(II)Br2 (1.5 equiv), acetonitrile, 75°C, 2 h, 87%; e) 5‐chloro‐3‐methyl‐[1,2,4] thiadiazole (1.2 equiv), Et3N (1.5 equiv), EtOH, 80°C, overnight, 97%; f) TFA (10 equiv), CH2Cl2, room temperature, overnight, 94%; g) intermediate 2 (1 equiv), sodium phenoxide (1.6 equiv), xantphos (0.16 equiv), Pd2dba.CHCl3 (0.08 equiv), 145°C, 45 min in microwave, 57%.
