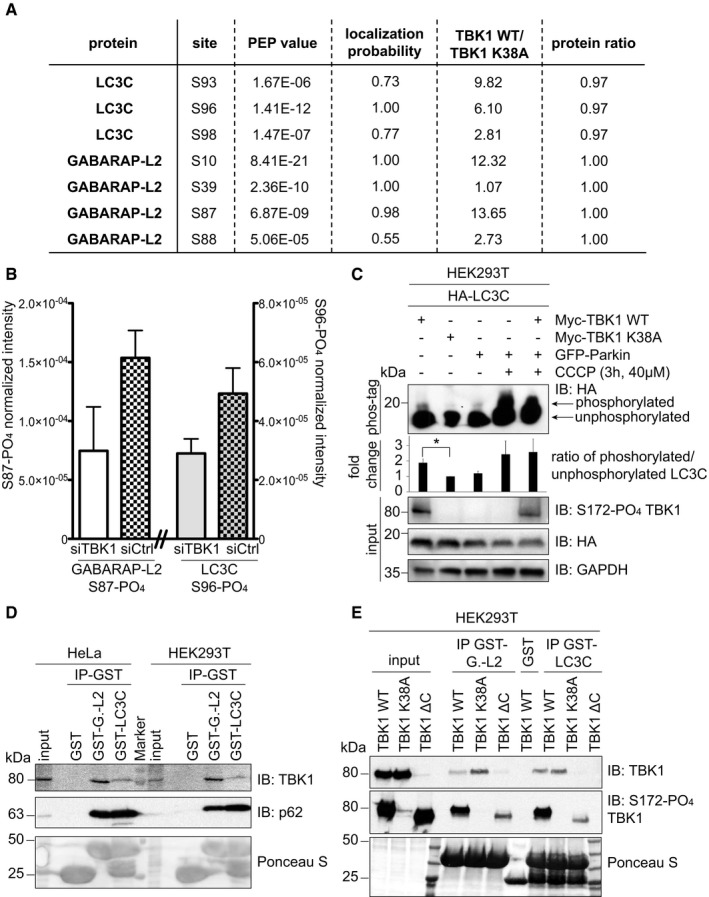
Figure 2. TBK1 phosphorylates and binds LC3C and GABARAP‐L2 in cells .
-
A, BIdentification of phosphosites by mass spectrometry following GFP‐LC3C or GFP‐GABARAP‐L2 immunoprecipitation. (A) TBK1 WT was overexpressed in heavy‐ and TBK1 kinase‐dead K38A was overexpressed in light‐labeled SILAC HEK293T cells. (B) HEK293T cells were treated with control or TBK1 siRNA and CCCP (3 h, 40 μM). Phosphosite intensities were normalized to total protein intensity. Data are presented as mean ± SD, n = 3 biological replicates.
-
CSDS–PAGE and Western blot of Phos‐tag™ gel with HEK293T cell lysates. Cells were transfected with HA‐LC3C, TBK1 WT or K38A, and GFP‐Parkin and left untreated or treated with CCCP (3 h, 40 μM) to induce mitophagy. The ratio of phosphorylated to unphosphorylated LC3C was quantified. Data are presented as mean ± SD, n = 3 biological replicates, *P < 0.05, as analyzed by Student's t‐test.
-
DSDS–PAGE and Western blot of HEK293T and HeLa cell lysates and GST‐LC3C or GST‐GABARAP‐L2 immunoprecipitations.
-
ESDS–PAGE and Western blot of HEK293T cell lysates transfected with full‐length TBK1, a C‐terminal truncation mutant (TBK1 ΔC), a kinase‐dead version (TBK1 K38A), and GST‐LC3C or GST‐GABARAP‐L2 immunoprecipitations.
Source data are available online for this figure.
