Figure EV3. Cyclin destruction assays using cell‐free Xenopus egg extracts.
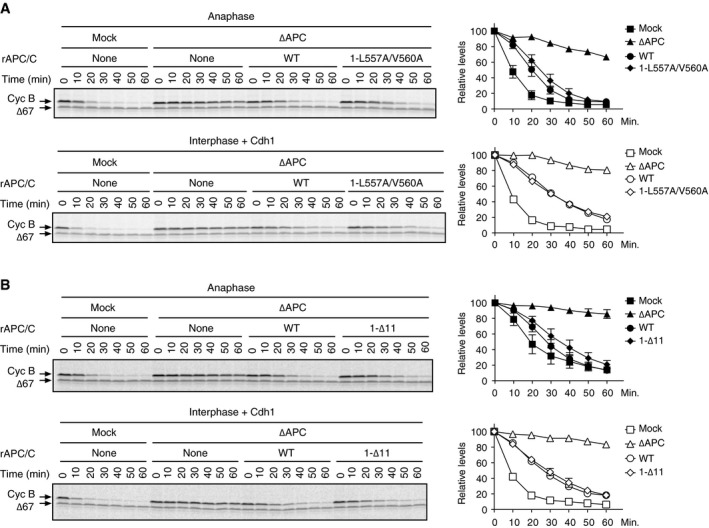
- (left panel) The B56 binding site mutant APC/C (1‐L557A/V560A) is less active than WT APC/C in cyclin destruction assays in an anaphase‐specific manner, whereas both activities are similar in interphase cyclin destruction assays. The purified recombinant WT APC/C or Apc1 mutant APC/C (1‐L557A/V560A) was incubated with APC/C‐depleted (∆APC) extract supplemented with CycB∆167 (anaphase, upper panel) or with Cdh1 (interphase, lower panel) at 23°C. 35S‐labelled cyclin B and a version of cyclin B lacking the N‐terminal 67 residues (Δ67, stable control) were used as substrates. Samples taken at indicated time points after addition of substrates were analysed by SDS–PAGE and autoradiography. (right panel) Quantification of cyclin destruction assays. Error bars, SEM from three independent experiments.
- (left panel) The activity of the B56 binding site mutant APC/C (1‐∆11) is less active than WT APC/C in anaphase cyclin destruction assay. The activity of recombinant APC/C (1‐∆11) was examined as in (A) (right panel) Quantification of cyclin destruction assays. Error bars, SEM from three independent experiments.
