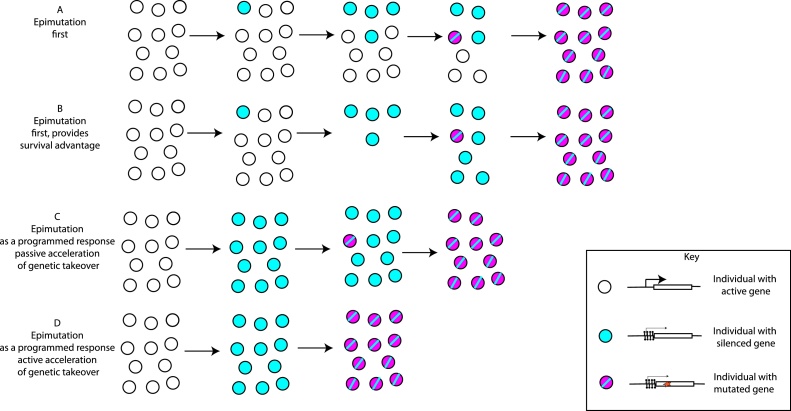
Fig. 2.
- An illustration of how epigenetic inheritance could affect DNA sequence evolution. This hypothetical example follows an epimutation caused by acquisition of DNA methylation that leads to silencing of a gene. The phenotypic effect of the inactivation of the gene is beneficial under certain environmental conditions that the population is exposed to. This example is for illustration only; the same process could apply to other types of epimutation with different molecular effects.
A- An epimutation arises in the population due to stochastic fluctuations (i.e. not promoted directly by environmental conditions). The effect of the epimutation is to silence a gene. Although it has a beneficial effect, the epimutation is unstable, because the same stochastic fluctuations can lead to the loss of the epimutation in individuals. However eventually a genetic mutation that inactivates the gene arises in the population. This has the same advantageous effect as the epimutation but is more stable and therefore eventually fixes in the population.
B- An epimutation that silences a gene arises in the population due to stochastic fluctuations as in A, but this time the epimutation provides a survival advantage under adverse conditions. Thus, although the epimutation is still unstable, the survival advantage that it poses gives enough time for the genetic mutation with the same effect to arise by chance and go to fixation within the population.
C- The epimutation that silences a gene occurs as a programmed response to the environment, thus occurs in every individual. Whilst the epimutation is unstable, this has the effect of accelerating the fixation of the inactivating mutation, which has the same effect as the epimutation because the population size remains high.
D- The epimutation that silences a gene occurs as a programmed response to the environment as in C. However in contrast to C, the epimutation has the effect of increasing the mutation rate at the same locus, through the fact that cytosine methylation increases the C–T transition rate (see text for molecular details of this effect). Thus the inactivating DNA sequence mutation occurs even more rapidly within the population than in C and then fixes in the population.