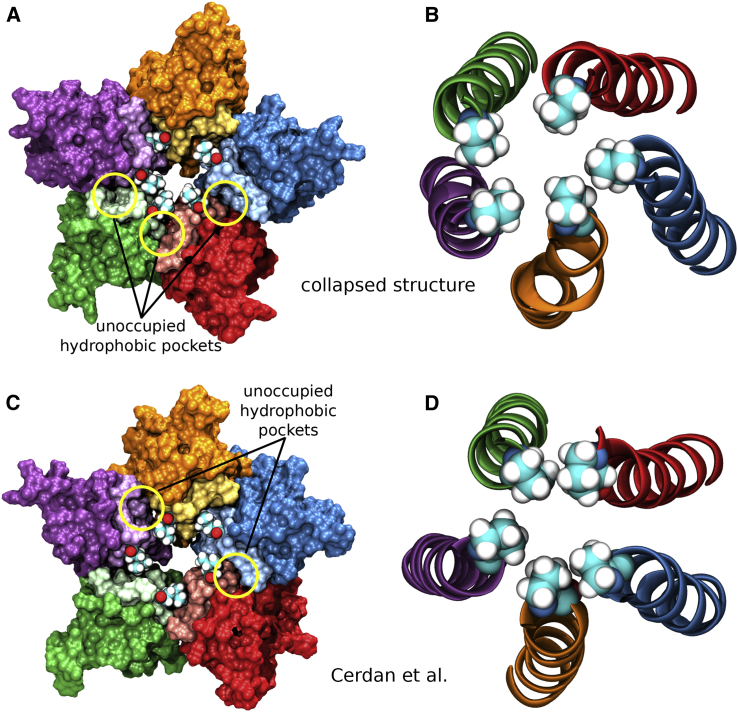
Figure 4.
Structural Features of the Collapsed State
(A and B) Representative structure from a collapsed simulation. (A) Although two hydrophobic pockets end up accommodating the corresponding leucine residues, the other three hydrophobic voids undergo a hydrophobic collapse, where the two subunits come closer together and push the leucine residues toward the pore center and decrease the pore radius significantly. (B) With the pore lining M2 helices coming closer together, the P-2′ residues undergo a hydrophobic collapse due to their strong hydrophobic interactions.
(C and D) Representative structure from a simulation by Cerdan et al. (2018). (C) Two hydrophobic pockets fail to accommodate their corresponding L9′ residue, leading to a much smaller pore radius. (D) Without all L9′ gate residues locking the structure into a stable open state, the P-2′ residues come so close to each other that their strong hydrophobic interactions lead to a hydrophobic collapse in the P-2′ ring region as well.