Abstract
Procollagen-lysine, 2-oxoglutarate 5-dioxygenase 2 (PLOD2), which affects collagen synthesis, is associated with breast cancer. The purpose of the study is to detect the expression of PLOD2 in breast cancer and to evaluate the correlation between PLOD2 and clinicopathologic characteristics and prognosis of patients with breast cancer. 50 paired samples including breast cancer tissues and adjacent non-tumor tissues were formalin-fixed and evaluated by immunohistochemistry. The results revealed that PLOD2 expression in breast cancer tissues was much higher than that in tissues adjacent to breast cancer. High expression of PLOD2 was positively associated with tumor stage (P = 0.003) and lymph node metastasis (P = 0.001). However, high expression of PLOD2 was negatively related to Ki-67 (P < 0.001) while positively related to progesterone receptor (PR) (P = 0.001). PLOD2 expression was positively related to the metastasis of breast cancer. Therefore, high expression of PLOD2 was identified as a poor prognostic biomarker for patients with breast cancer. These results suggest a novel molecular mechanism in breast cancer tumorigenesis, thus providing a potential therapeutic target of breast cancer.
Keywords: Breast cancer, PLOD2, metastasis
Introduction
Breast cancer, according to GLOBOCAN, is the most common malignancy in women worldwide. The incidence and mortality of breast cancer are increasing in the past few years, resulting in a serious threat to women’s health [1,2]. In China and many developed countries, the treatment of breast cancer has improved in the past 20 years [3]. The clinicopathologic factors, such as lymph node metastasis, tumor differentiation, and clinical stage, are closely related to prognosis of patients with breast cancer [4]. Although human epidermal growth factor receptor-2 (HER-2), estrogen receptor (ER) and progesterone receptor (PR) have been proved a reference for the prognosis of breast cancer, more biomarkers are needed for the prediction of the clinical prognosis [5]. So it is valuable to understand its molecular mechanisms and find more biomarkers for new therapeutic strategies of breast cancer.
Collagen provides a prolific protein in the extracellular matrix (ECM) for its assemblage and linearly collagen is known as the “highway” for cancer cell invasion and migration [6,7]. In breast cancer, collagen shows a cross-linked state of the fiber bundles, which activate integrin signaling and promote the formation of focal adhesions to migrate tumor cells to the ECM [8]. Living imaging techniques also provideevidence that breast cancer cells can move rapidly along the fiber bundles [9]. Increasing density of collagen fibers is associated with high rate of recurrence and poor prognosis of breast cancer [6,10].
Lysyl hydroxylases 2, encoded by the procollagen-lysine, 2-oxoglutarate 5-dioxygenase 2 (PLOD2), mediates the conformation of collagen cross-links. It has beenm confirmed that PLOD2 hydroxylates telopeptidyl lysine residues and results in less lysine aldehyde-derived cross-links (LCCs) but more hydroxylysine aldehyde-derived collagen cross-links (HLCCs). HLCCs lead to accumulated excessive collagen to form collagen fibrosis which enhances breast cancer cell invasion and metastasis [11].
PLOD2 has been reported overexpressed in many cancers, such as oral cancer, lung cancer, cervical cancer, sarcoma, bladder cancer, liver cancer, glioblastoma multiforme and renal cell carcinoma [12-19]. Previous studies have confirmed that the expression of PLOD2 is regulated by multiplex factors, such as FOXA1, TGF-β, microRNA-26a/b and HIF-1α. In non-small-cell lung cancers (NSCLC), FOXA1 directly regulates PLOD2 through PI3K/AKT-FOXA1 axis to trigger the transcription of PLOD2 [13]. PLOD2 induced by hypoxia and TGF-β1 improves the invasion and migration of cervical cancer cells by promoting the epithelial-to-mesenchymal transition (EMT) and the formation of focal adhesions [20]. In renal cell carcinoma and bladder cancer, microRNA-26a and microRNA-26ab directly downregulate PLOD2 respectively, contributing to the favorable prognosis of these two cancers [16,19]. In the continuous hypoxic environment of breast cancer, HIF-1α activates transcription of PLOD2 gene and increases PLOD2 protein levels for dozens of hours [8]. Also, the mechanism of HIF-1a regulating PLOD2 has been well identified in vitro [15,21].
However, the dynamic changes of PLOD2 expression in the process of breast cancer development have not been reported.
In this study, in order to confirm whether PLOD2 might be a potential marker for the diagnosis and prognosis of breast cancer, expression of POLD2 was examined in breast cancer tissues and adjacent normal tissues. The relationship between the expression of PLOD2 and the clinicopathologic characteristics in breast cancer was assessed by immunohistochemistry.
Patients and methods
Patients and tissue specimens
Breast cancer tissues and adjacent normal tissues were excised from 50 paired female patients who were diagnosed with invasive breast carcinoma at Zhongda Hospital, between 2015 and 2018, were collected for immunohistochemistry analysis in the present study. All specimens were formalin-fixed and paraffin-embedded. None of the patients had received radiotherapy or neoadjuvant chemotherapy before surgery. Clinicopathologic data of these patients were collected, including primary tumor size, tumor stage, nuclear grade, the status of ER, PR, HER-2, Ki-67 and lymph nodes metastasis. The tumor stages were classified according to the American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the TNM and the nuclear grades were classified according to the Nottingham combined histologic grade [22,23]. Specimens with more than 14% of Ki-67 tumor cells were considered Ki-67 positive [24]. Written informed consent was obtained before their surgery.
Immunohistochemical analysis
All surgical specimens were fixed by 10% formalin and embedded in paraffin and used for the evaluation of PLOD2 expression. The specimens were cut into 4 μm sections and each tissue section was deparaffinized in xylenes and rehydrated through a graded ethanol series.
The sections were immersed in citrate buffer (pH 6.0) and placed in a pressure cooker with maximum pressure for 2.5 min. The sections were taken out and naturally cooled to room temperature for 10 min and washed 3 times with phosphate buffered saline (PBS).
3% hydrogen peroxide blocked endogenous peroxidase activity in sections for 15 min. The sections were washed in PBS and incubated with the rabbit polyclonal anti-PLOD2 antibody (diluted 1:200; Sigma, USA) overnight at 4°C. Next, the sections were incubated with a secondary antibody for 30 min at 30°C after washing with PBS. Finally, color reaction was carried out with 3, 3’-diaminobenzidine (DAB).
Staining evaluation
The PLOD2 protein staining was scored independently by two experienced pathologists blind to the clinicopathologic characteristics, and they finally reached an agreement on the staining result.
PLOD2 expression was evaluated according to the staining intensity and percentage of positive cells. The PLOD2 expression was assessed for staining intensity in 4 grades (0-3): (0 = no staining; 1 = weak; 2 = moderate; 3 = strong) and the percentage of positively stained cells in 4 grades (0-4) (0 = 0% of stained cells; 1 = ≤ 10% of stained cells; 2 = 10-50% of stained cells; 3 = 50-80% of stained cells; 4 ≥ 80% of stained cells). For statistical analysis of staining evaluation, two types of scores were multiplied. The PLOD2 expression was considered low if scores were from 0 to 7 and scores of 8 to 12 were considered as high expression of PLOD2.
Statistical analysis
All statistical analyses were carried out using SPSS software (SPSS 20.0). t-test was used to access statistically significant differences between breast cancer tissue group and tissue adjacent to breast cancer group. Chi-square test was used to analyze the relationship between PLOD2 expression and clinicopathologic characteristics. P values < 0.05 were considered significant.
Results
PLOD2 expression in breast cancer
Figure 1 showed that PLOD2 was localized in the cytoplasm. According to the grading standard described above, 27 out of 50 (54.0%) samples had strong or high expressed PLOD2 and the remaining 23 (46.0%) samples had none or low expression PLOD2 in breast cancer tissues (Figure 1C and 1D; Table 1). However, only 5 out of 50 (10.0%) samples had strong or high expressed PLOD2 among those matched tissues adjacent to breast cancer (Figure 1A and 1B; Table 1). Immunochemical data confirmed that the expression of PLOD2 in breast cancer tissues was higher than that of tissues adjacent to breast cancer (P < 0.001).
Figure 1.
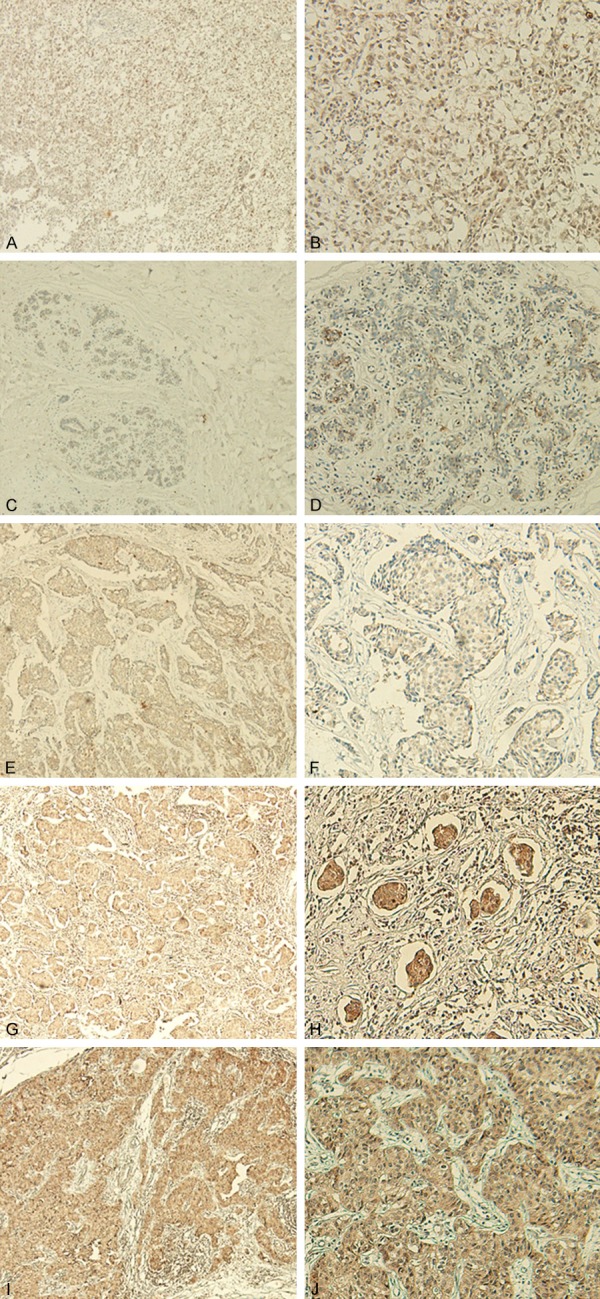
Immunohistochemical expression of PLOD2 in breast cancer tissues and tissues adjacent to breast cancer. (A, B) Negative staining of PLOD2 in tissue adjacent to breast cancer (magnification: A × 40 and B × 100). (C, E, G, I) Respectively represent negative, weak, moderate, and strong staining of PLOD2 in the cytoplasm of breast cancer tissues (× 40 magnification). (D, F, H, J) Respectively represent negative, weak, moderate and strong staining of PLOD2 in the cytoplasm of breast cancer tissues (× 100 magnification).
Table 1.
Relationship between expression of PLOD2 and patient characteristics
| Group | Number | PLOD2 | P-value | |
|---|---|---|---|---|
|
| ||||
| Low | High | |||
| Breast cancer tissue | 50 | 23 (46.0%) | 27 (54.0%) | |
| Tissue adjacent to breast cancer | 50 | 45 (90.0%) | 5 (10.0%) | < 0.001 |
| Breast cancer tissue | 50 | 23 (46.0%) | 27 (54.0%) | |
| Normal breast tissue | 50 | 48 (96.0%) | 2 (4.0%) | < 0.001 |
| Tissue adjacent to breast cancer | 50 | 45 (90.0%) | 5 (10.0%) | |
| Normal breast tissue | 50 | 48 (96.0%) | 2 (4.0%) | NS |
P values were calculated by χ2 test; NS represented not significant.
Positive PLOD2 expression was relevant to tumor stage
In this study, 14/27 (51.9%) specimens with high expressed PLOD2 were positive for the tumor stage; however, 2/23 (8.71%) specimens with low expressed PLOD2 were positive for the tumor stage. In conclusion, high expressed PLOD2 was positively associated with the tumor stage in breast cancer (P = 0.003) (Table 2).
Table 2.
Relationship between expression of PLOD2 and patient characteristics
| Characteristics | Number | PLOD2 | P value | |
|---|---|---|---|---|
|
| ||||
| Low | High | |||
| Total number | 50 | 23 | 27 | |
| Age (years) | ||||
| < 50 | 21 | 10 | 11 | NS |
| ≥ 50 | 29 | 13 | 16 | |
| Tumor size (cm) | ||||
| < 2 | 31 | 14 | 17 | NS |
| ≥ 2 | 19 | 9 | 10 | |
| Tumor stage | ||||
| I-II | 34 | 21 | 13 | 0.003 |
| III-IV | 16 | 2 | 14 | |
| ER status | ||||
| Negative | 20 | 10 | 10 | NS |
| Positive | 30 | 13 | 17 | |
| PR status | ||||
| Negative | 24 | 17 | 7 | 0.001 |
| Positive | 26 | 6 | 20 | |
| HER-2 status | ||||
| Negative | 18 | 6 | 12 | NS |
| Positive | 32 | 17 | 15 | |
| Ki-67 status | ||||
| Negative | 21 | 3 | 18 | < 0.001 |
| Positive | 29 | 20 | 9 | |
| Lymph nodal status | ||||
| Negative | 30 | 20 | 10 | 0.001 |
| Positive | 20 | 3 | 17 | |
P values were calculated by χ2 test; NS represented not significant.
Improved PLOD2 expression was associated with Ki-67
The aggressiveness of breast cancer is related to proliferation of tumor cells, and Ki-67 is a proliferation marker in breast cancer which can be confirmed through many studies [25]. In this study, 9/27 (33.3%) samples with high expression of PLOD2 were positive for expression of Ki-67, but 20/23 (87.0%) samples with low expression of PLOD2 were positive for expression of Ki-67. This result shows that low expression of PLOD2 is related to positive expression of Ki67 in breast cancer (P < 0.001) (Table 2).
High expression of PLOD2 is related to lymph node metastasis
It is of interest to analyze the association between PLOD2 expression and lymph node metastasis for its significance for breast cancer prognosis. 17 (63.0%) patients had lymph node metastasis in the 27 breast cancer patients with high expression of PLOD2; however, only 3 (13.0%) patients had lymph node metastasis among the 23 breast cancer patients with low expression of PLOD2. It was important to find lymph node metastasis increased with high expression of PLOD2 in these breast cancer samples (P = 0.001) (Table 2).
Positive PLOD2 expression is relevant to PR
In this study, 20/27 (74.1%) specimens with high expression of PLOD2 were positive for the expression of PR; however, 9/23 (39.1%) specimens with low expression of PLOD2 were positive for the expression of PR. In conclusion, high expression of PLOD2 was positively associated with the expression of PR in breast cancer (P = 0.001) (Table 2).
Discussion
The incidence of breast cancer is the highest among all cancers and breast cancer is a main cause of cancer mortality second only to lung cancer in women. The metastasis of breast cancer involves complex molecular mechanisms. However, the traditional cancer staging has limitations in judging the prognosis. Therefore, it is of great value to detect a new biomarker to assess the prognosis of breast cancer.
PLOD2 has been proven to be relevant to collagen crosslinking by inducing collagen reorganization [11]. Many studies have also reported that tumor cells preferentially moved along fiber bundles, which could be considered as a “highway” for tumor cell invasion and metastasis [6,9,26]. So, PLOD2 can be considered as an important biomarker for metastasis and prognosis of breast cancer.
In our study, we found that PLOD2 expression was notably higher in breast cancer tissues than that in tissues adjacent to breast cancer. Moreover, high expression of PLOD2 was positively associated with tumor stage (P = 0.003) and lymph node metastasis (P = 0.001), suggesting that PLOD2 was able to enhance metastasis by acting as an oncogene, especially related to the local and distant migration of breast cancer cells. Nevertheless, high expression of PLOD2 was negatively related to Ki-67 (P < 0.001) and positively related to PR (P = 0.001). Many studies have demonstrated that Ki-67 promotes cell proliferation and influences the prognosis of breast cancer, especially in HER2-positive (non-luminal) breast cancer [27,28]. Ki-67 and PR are both proven to be associated with the proliferation of breast cancer cells to enhance breast cancer metastasis. Previous studies have found that the expression of Ki-67 is inversely related to PR in breast cancer [29]. However, we cannot explain the reason that PLOD2 is positively correlated with cell migration but negatively correlated with cell proliferation. So far, no evidence has confirmed that PLOD2 is associated with the proliferation of breast cancer cells.
We conjecture that PLOD2 may play vital roles in carcinoma progression and metastasis by interacting with some tumor-promoting factors in the tumor miroenvironment (TME). The metastasis of breast cancers is associated with the TME [30]. In the hypoxic environment, hypoxia-inducible factor 1 (HIF-1) activates the transcription of PLOD2 to promote collagen fibrosis [8]. In epithelial tumors of Kras (LA1) mice, cancer associated fibroblasts (CAFs) formed networks, tumor cell aggregates responded to signals of CAFs and move to the networks [31]. However, the consumption of PLOD2 expression in CAFs reduced the formation of networks and the dissociation of tumor aggregates. This means CAFs induce collagen cross-linking switch to influence metastasis. These studies indicate significant roles of PLOD2 interacting with molecules in the metastasis of breast cancer. We believe that PLOD2 can indirectly inhibit the proliferation of tumor cells and promote the migration of tumor cells by interacting with some factors in the TME. Further experiments are needed to test this conjecture.
There are some limitations in our study. The number of specimens was relatively few, and only invasive breast cancer specimens were collected. If the specimens are adequate, the expression of PLOD2 in ductal carcinoma in situ (DCIS) and in invasive carcinoma (IDC), and the expression of PLOD2 in different molecular types of IDC should both be compared. This is what our next experiment will do next.
There are limitations in the targeted therapy of breast cancer, and only one targeted therapy drug for patients of HER-2-positive. Also, there is no targeted drug for the metastasis of breast cancer. Based on this study, we considered whether PLOD2 inhibitors combined with other drugs can be used in metastatic breast cancer to prolong survival of breast cancer patients.
In conclusion, this study clarifies the role of PLOD2 protein in breast cancer metastasis. High expression of PLOD2 is positively associated with tumor stage and lymph node metastasis. PLOD2 may be a better indication of breast cancer metastasis in the movement of tumor cells. However, it is still unknown how collagen fiber bundles affect migration and invasion of breast cancer cells. More experiments will be conducted to solve this problem in the future.
Acknowledgements
This work was supported by National Natural Science Foundation of China (grant numbers 81573456, 81502287). This study was conducted with approval from the Ethics Committee of The Affiliated Zhongda Hospital. Written informed consent was obtained from all participants. We gratefully acknowledge our colleagues at Pathology department, Breast Disease Center, Affiliated Zhongda Hospital for their and technical supports.
Written informed consent was obtained from all participants.
Disclosure of conflict of interest
None.
References
- 1.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66:31–42. doi: 10.3322/caac.21320. [DOI] [PubMed] [Google Scholar]
- 3.Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, Bannon F, Ahn JV, Johnson CJ, Bonaventure A, Marcos-Gragera R, Stiller C, Azevedo e Silva G, Chen WQ, Ogunbiyi OJ, Rachet B, Soeberg MJ, You H, Matsuda T, Bielska-Lasota M, Storm H, Tucker TC, Coleman MP CONCORD Working Group. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, Martiat P, Fox SB, Harris AL, Liu ET. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A. 2003;100:10393–10398. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuzick J, Dowsett M, Pineda S, Wale C, Salter J, Quinn E, Zabaglo L, Mallon E, Green AR, Ellis IO, Howell A, Buzdar AU, Forbes JF. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the genomic health recurrence score in early breast cancer. J. Clin. Oncol. 2011;29:4273–4278. doi: 10.1200/JCO.2010.31.2835. [DOI] [PubMed] [Google Scholar]
- 6.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4:38. doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y, Guo H, Terajima M, Banerjee P, Liu X, Yu J, Momin AA, Katayama H, Hanash SM, Burns AR, Fields GB, Yamauchi M, Kurie JM. Lysyl hydroxylase 2 is secreted by tumor cells and can modify collagen in the extracellular space. J Biol Chem. 2016;291:25799–25808. doi: 10.1074/jbc.M116.759803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilkes DM, Bajpai S, Wong CC, Chaturvedi P, Hubbi ME, Wirtz D, Semenza GL. Procollagen lysyl hydroxylase 2 is essential for hypoxia-induced breast cancer metastasis. Mol Cancer Res. 2013;11:456–466. doi: 10.1158/1541-7786.MCR-12-0629. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, Segall JE, Pollard JW, Condeelis J. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–2656. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 10.Hasebe T, Tsuda H, Tsubono Y, Imoto S, Mukai K. Fibrotic focus in invasive ductal carcinoma of the breast: a histopathological prognostic parameter for tumor recurrence and tumor death within three years after the initial operation. Jpn J Cancer Res. 1997;88:590–599. doi: 10.1111/j.1349-7006.1997.tb00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamauchi M, Sricholpech M. Lysine post-translational modifications of collagen. Essays Biochem. 2012;52:113–133. doi: 10.1042/bse0520113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reis PP, Waldron L, Goswami RS, Xu W, Xuan Y, Perez-Ordonez B, Gullane P, Irish J, Jurisica I, Kamel-Reid S. mRNA transcript quantification in archival samples using multiplexed, color-coded probes. BMC Biotechnol. 2011;11:46. doi: 10.1186/1472-6750-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du H, Chen Y, Hou X, Huang Y, Wei X, Yu X, Feng S, Wu Y, Zhan M, Shi X, Lin S, Lu L, Yuan S, Sun L. PLOD2 regulated by transcription factor FOXA1 promotes metastasis in NSCLC. Cell Death Dis. 2017;8:e3143. doi: 10.1038/cddis.2017.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajkumar T, Sabitha K, Vijayalakshmi N, Shirley S, Bose MV, Gopal G, Selvaluxmy G. Identification and validation of genes involved in cervical tumourigenesis. BMC Cancer. 2011;11:80. doi: 10.1186/1471-2407-11-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisinger-Mathason TS, Zhang M, Qiu Q, Skuli N, Nakazawa MS, Karakasheva T, Mucaj V, Shay JE, Stangenberg L, Sadri N, Puré E, Yoon SS, Kirsch DG, Simon MC. Hypoxia-dependent modification of collagen networks promotes sarcoma metastasis. Cancer Discov. 2013;3:1190–1205. doi: 10.1158/2159-8290.CD-13-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyamoto K, Seki N, Matsushita R, Yonemori M, Yoshino H, Nakagawa M, Enokida H. Tumour-suppressive miRNA-26a-5p and miR-26b-5p inhibit cell aggressiveness by regulating PLOD2 in bladder cancer. Br J Cancer. 2016;115:354–363. doi: 10.1038/bjc.2016.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noda T, Yamamoto H, Takemasa I, Yamada D, Uemura M, Wada H, Kobayashi S, Marubashi S, Eguchi H, Tanemura M, Umeshita K, Doki Y, Mori M, Nagano H. PLOD2 induced under hypoxia is a novel prognostic factor for hepatocellular carcinoma after curative resection. Liver Int. 2012;32:110–118. doi: 10.1111/j.1478-3231.2011.02619.x. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y, Zhang L, Wei Y, Zhang X, Xu R, Han M, Huang B, Chen A, Li W, Zhang Q, Li G, Wang J, Zhao P, Li X. Procollagen-lysine 2-oxoglutarate 5-dioxygenase 2 promotes hypoxia-induced glioma migration and invasion. Oncotarget. 2017;8:23401–23413. doi: 10.18632/oncotarget.15581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurozumi A, Kato M, Goto Y, Matsushita R, Nishikawa R, Okato A, Fukumoto I, Ichikawa T, Seki N. Regulation of the collagen cross-linking enzymes LOXL2 and PLOD2 by tumor-suppressive microRNA-26a/b in renal cell carcinoma. Int J Oncol. 2016;48:1837–1846. doi: 10.3892/ijo.2016.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu F, Zhang J, Hu G, Liu L, Liang W. Hypoxia and TGF-β1 induced PLOD2 expression improve the migration and invasion of cervical cancer cells by promoting epithelial-to-mesenchymal transition (EMT) and focal adhesion formation. Cancer Cell Int. 2017;17:54. doi: 10.1186/s12935-017-0420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noda T, Yamamoto H, Takemasa I, Yamada D, Uemura M, Wada H, Kobayashi S, Marubashi S, Eguchi H, Tanemura M, Umeshita K, Doki Y, Mori M, Nagano H. PLOD2 induced under hypoxia is a novel prognostic factor for hepatocellular carcinoma after curative resection. Liver Int. 2012;32:110–118. doi: 10.1111/j.1478-3231.2011.02619.x. [DOI] [PubMed] [Google Scholar]
- 22.Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, Borgen PI, Clark G, Edge SB, Hayes DF, Hughes LL, Hutter RV, Morrow M, Page DL, Recht A, Theriault RL, Thor A, Weaver DL, Wieand HS, Greene FL. Revision of the American joint committee on cancer staging system for breast cancer. J. Clin. Oncol. 2002;20:3628–3636. doi: 10.1200/JCO.2002.02.026. [DOI] [PubMed] [Google Scholar]
- 23.Edge SB, Compton CC. The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 24.Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, Perou CM, Ellis MJ, Nielsen TO. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haroon S, Hashmi AA, Khurshid A, Kanpurwala MA, Mujtuba S, Malik B, Faridi N. Ki67 index in breast cancer: correlation with other prognostic markers and potential in pakistani patients. Asian Pac J Cancer Prev. 2013;14:4353–4358. doi: 10.7314/apjcp.2013.14.7.4353. [DOI] [PubMed] [Google Scholar]
- 26.Han W, Chen S, Yuan W, Fan Q, Tian J, Wang X, Chen L, Zhang X, Wei W, Liu R, Qu J, Jiao Y, Austin RH, Liu L. Oriented collagen fibers direct tumor cell intravasation. Proc Natl Acad Sci U S A. 2016;113:11208–11213. doi: 10.1073/pnas.1610347113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kontzoglou K, Palla V, Karaolanis G, Karaiskos I, Alexiou I, Pateras I, Konstantoudakis K, Stamatakos M. Correlation between Ki67 and breast cancer prognosis. Oncology. 2013;84:219–225. doi: 10.1159/000346475. [DOI] [PubMed] [Google Scholar]
- 28.Sun JZ, Chen C, Jiang G, Tian WQ, Li Y, Sun SR. Quantum dot-based immunofluorescent imaging of Ki67 and identification of prognostic value in HER2-positive (non-luminal) breast cancer. Int J Nanomedicine. 2014;9:1339–1346. doi: 10.2147/IJN.S58881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J. Clin. Oncol. 2005;23:7212–7220. doi: 10.1200/JCO.2005.07.501. [DOI] [PubMed] [Google Scholar]
- 30.Soysal SD, Tzankov A, Muenst SE. Role of the tumor microenvironment in breast cancer. Pathobiology. 2015;82:142–152. doi: 10.1159/000430499. [DOI] [PubMed] [Google Scholar]
- 31.Pankova D, Chen Y, Terajima M, Schliekelman MJ, Baird BN, Fahrenholtz M, Sun L, Gill BJ, Vadakkan TJ, Kim MP, Ahn YH, Roybal JD, Liu X, Parra Cuentas ER, Rodriguez J, Wistuba II, Creighton CJ, Gibbons DL, Hicks JM, Dickinson ME, West JL, Grande-Allen KJ, Hanash SM, Yamauchi M, Kurie JM. Cancer-associated fibroblasts induce a collagen cross-link switch in tumor stroma. Mol Cancer Res. 2016;14:287–295. doi: 10.1158/1541-7786.MCR-15-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]


