Fig. 3.
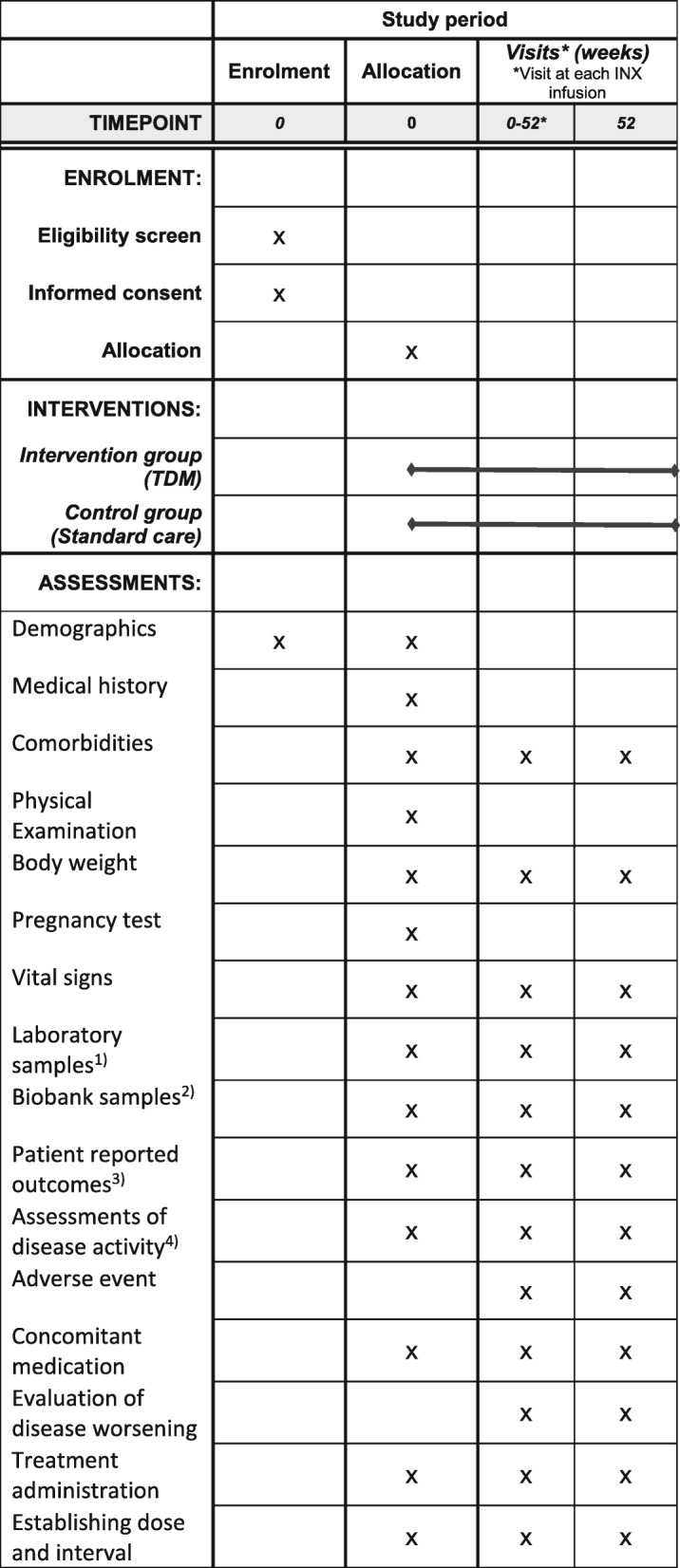
Schedule of enrolment, interventions and assessments NOR-DRUM B. 1. Laboratory samples: haemoglobin, white blood cells with differentials, platelet counts, ALT, albumin, creatinine, CRP, ESR, fecal calprotectin (IBD only).2. Biobank samples: serum and full blood at baseline, serum at following visits.3. Patient-reported outcomes: patient global assessment of disease activity (NRS), EQ-5D, SF-36, WPAI-GH, RA: M-HAQ, RAID, PsA: M-HAQ, PsAID, DLQI, SpA: M-HAQ, BASDAI, UC and CD: IBDQ, Psoriasis: DLQI.4. Assessments of disease activity: Nurse/investigator global assessment of disease activity (VAS), RA: DAS28, CDAI, SDAI, PsA: DAS28, DAPSA, SpA: ASDAS, UC: Partial Mayo score, CD: HBI, Psoriasis: PASI. ALT alanine aminotransferase, ASDAS Ankylosing Spondylitis Disease Activity Score, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, CD Crohn’s disease, CDAI Clinical Disease Activity Index, CRP C-reactive protein, DAS28 Disease Activity Score using 28 joints, DLQI Dermatology Life Quality Index, ESR erythrocyte sedimentation rate, HBI Harvey-Bradshaw Index, IBD inflammatory bowel diseases, IBDQ Inflammatory Bowel Disease Questionnaire, INX infliximab, MHAQ Modified Health Assessment Questionnaire, PASI Psoriasis Area and Severity Index, PMS partial Mayo score, Ps psoriasis, PsA psoriatic arthritis, PsAID Psoriatic Arthritis Impact of Disease, SDAI Simplified Disease Activity Index, RA rheumatoid arthritis, RAID Rheumatoid Arthritis Impact of Disease, SF-36 Short Form Health Survey, SpA spondyloarthritis, UC ulcerative colitis, VAS visual analogue scale
