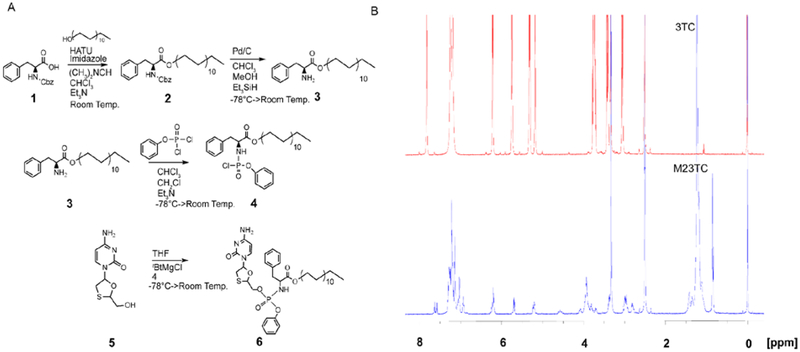
Figure 1. Synthesis and characterization of M23TC.
(A) Synthesis of aryl amino phosphochloridate masking group as previously described [16]. Briefly, Cbz protected phenylalanine (1) was reacted with docosanol to give (2), followed by Cbz cleavage to yield 3. Benzyl dichlorophosphonate was then reacted with 3 to give the aryl amino phosphochloridate masking group (4). The masking group was conjugated to 3TC (5) using tBuMgCl base in anhydrous THF to form M23TC in 65% yield (6). (B) Proton NMR spectra of 3TC (in red) and M23TC (in blue): 1H-NMR (M23TC), (500 MHz, (CD3)2S=O): 7.88 (d, J = 7.3 Hz 1H), 7.60 (app. d, J = 7.6 Hz 1H), 7.10–7.35 (m, 8 H), 7.03 (app. d, J = 7.9 Hz 2H), 6.15–6.29 (m, 1H), 5.65–5.75 (m, 1H), 5.19–5.29 (m, 1H), 3.92 (br, 5H), 3.35–3.48 (m, 4H), 2.90–3.02 (m, 1H), 2.75–2.85 (m, 1H), 1.42 (br, 2H), 1.14–1.33 (m, 38H), 0.85 (br, 3H)