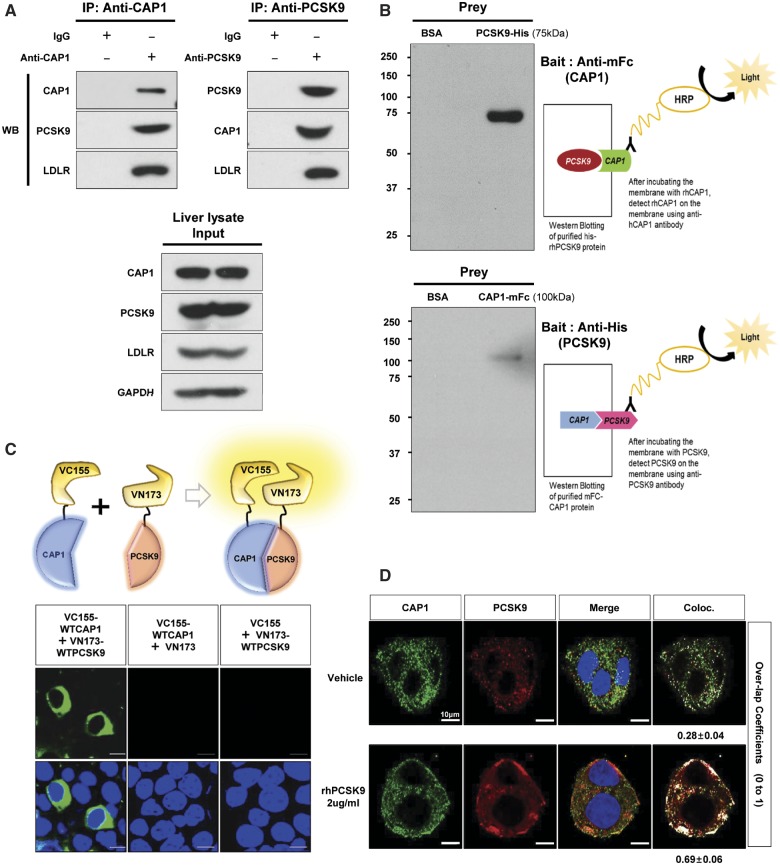
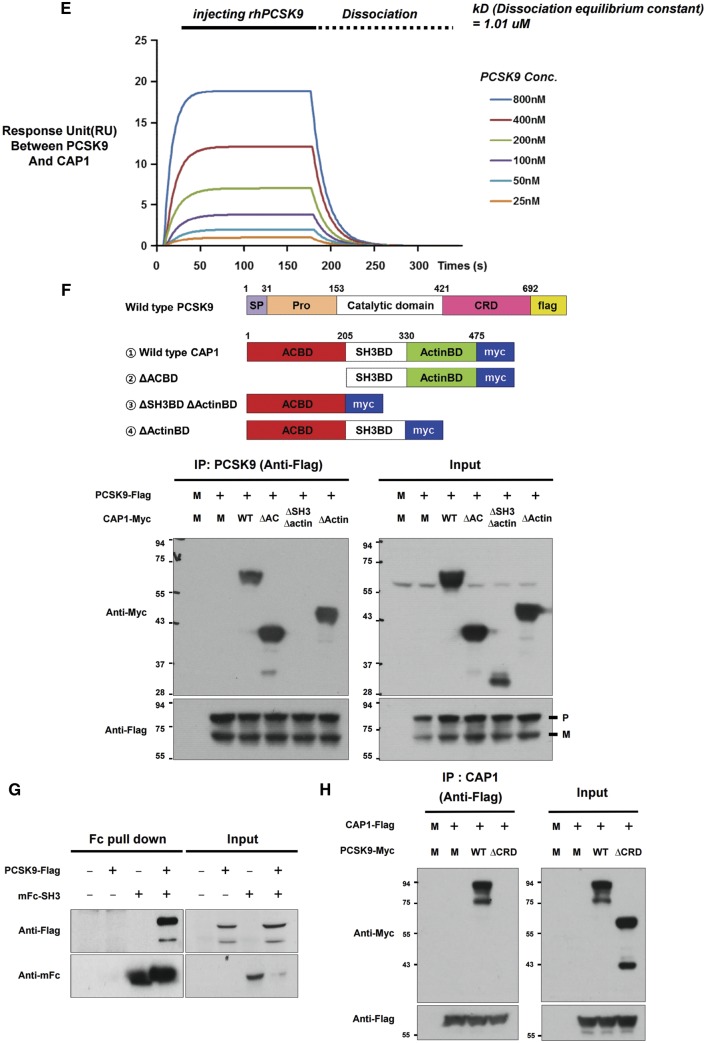
Figure 1.
CAP1 directly interacts with PCSK9. (A) Immunoprecipitation of endogenous CAP1 or PCSK9 from liver lysates of C57/BL6 wild-type mice. (B) Far-western blot analysis of mFc-hCAP1 and hPCSK9-His. (C) Bimolecular fluorescence complementation assay visualizing the interaction between hCAP1 and hPCSK9 in living cells. Green fluorescence was observed when CAP1 and PCSK9 fused to each fragment (pVC155 and pVN173) and were both expressed (left panel). However, when human CAP1 or PCSK9 was expressed alone, no fluorescence was detected (middle and right panel, respectively). (D) Colocalization of CAP1 and PCSK9 as determined by immunofluorescent staining of HepG2 cells exogenously treated with recombinant hPCSK9. Overlap coefficients: mean ± standard deviation. (E) Direct binding assay between rhPCSK9 and CAP1 using surface plasmon resonance. The equilibrium dissociation constant was 1.01 µM. (F) Coimmunoprecipitation of hPCSK9-Flag and wild-type CAP1 or each CAP1 mutant in HEK 293 cells. Pro-PCSK9 (P) and mature PCSK9 (M) are indicated with bold line. (G) Coimmunoprecipitation of hPCSK9-Flag and the SH3BD of CAP1 in HEK 293 cells showing that the SH3BD is sufficient for binding to CAP1. (H) Coimmunoprecipitation of wild-type hCAP1 with wild-type PCSK9 or the CRD deletion PCSK9 mutant in HEK 293 cells showing that PCSK9 binds CAP1 via the CRD.