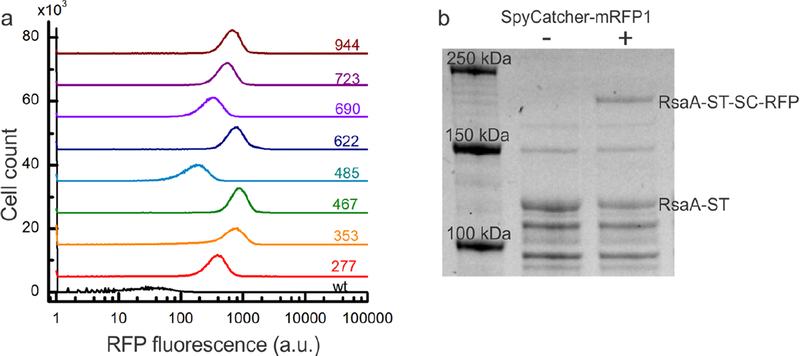
Figure 4.
SpyCatcher protein fusions covalently bind to RsaA-SpyTag with variable occupancy according to the SpyTag location. (a) Flow cytometry histograms of RFP fluorescence per cell for strains expressing wild-type RsaA (black) and RsaA-SpyTag (colored lines) incubated with SpyCatcher-mRFP1 for 1 h. Baselines are offset for clarity. All eight strains displaying RsaA-SpyTag show an increase in the intensity of RFP fluorescence over the negative control with their intensity varying based on where SpyTag is inserted within RsaA. (b) SDS-PAGE of whole cell lysates from the rsaA467:SpyTag strain incubated for 24 h without (lane 2) and with (lane 3) SpyCatcher-mRFP1 protein. Appearance of a higher molecular weight band only in the reaction containing SpyCatcher-mRFP1 indicates covalent binding to RsaA-SpyTag.