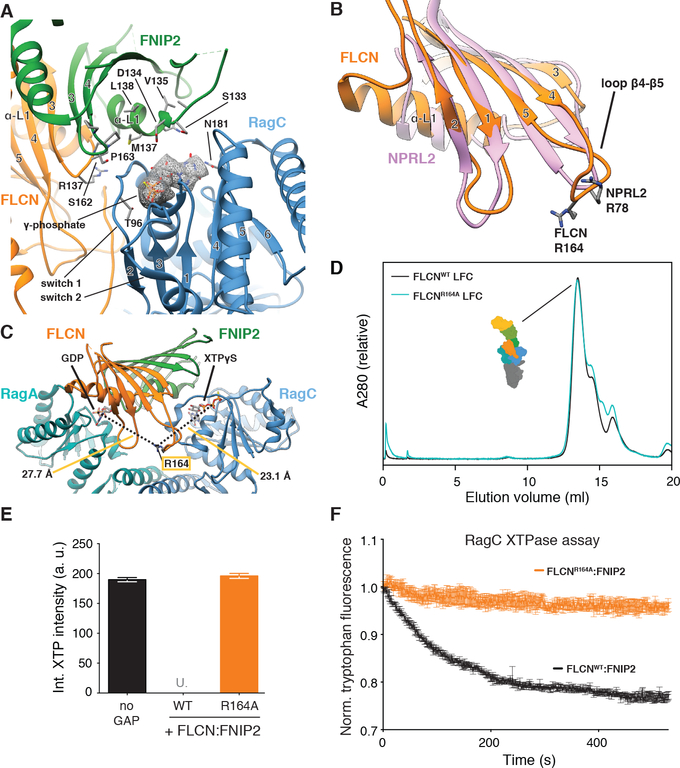
Fig. 5. FLCN Arg164 mediates GAP activity and is in a GAP-incompetent position in the LFC.
(A) Nucleotide-binding pockets and interaction with FLCN:FNIP2 (orange, green) of the RagC G domain (blue) in the LFC. XTPγS is highlighted by the according cryo-EM density and stick representation. FLCN:FNIP2 residues in the interface are labeled and side chains are shown as sticks. The γ-phosphate of XTPγS locking the switch 1 and switch 2 region of RagC is indicated. (B) Overlay of the FLCN (orange) and NPRL2 (rose) longin domains. α-helices α3 and α4 are omitted for clarity. NPRL2 arginine finger (R78) and FLCN R164 in loop β4-β5 are represented as sticks. (C) Position of FLCN R164 in the LFC. Distances to the β-phosphate of GDP (RagA) and XTPγS (RagC) are indicated. (D) SEC elution profile of the LFC (Ragulator-RagAGDP:RagCXTPγS-FLCN:FNIP2) containing FLCNWT (black) or mutant FLCNR164A (cyan). (E) HPLC-based GTPase assay assessing GAP activity of FLCNWT:FNIP2 or FLCNR164A:FNIP2 (orange) on a RagAGTP:RagCXTP substrate by integrating the remaining XTP nucleotide signal. Plotted are mean +/− SD. N=3. “U.” indicates that remaining XTP signal was undetectable by HPLC. (F) Tryptophan fluorescence XTPase assay with FLCN:FNIP2 mutants. RagAGTP:RagCXTP is incubated with FLCNWT:FNIP2 (black) or FLCNR164:FNIP2 (orange). Plotted are mean +/− SEM. N=3.