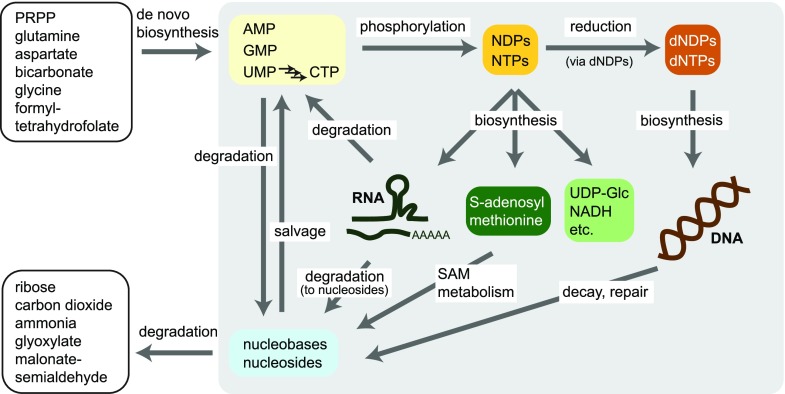
Figure 2.
Schematic overview of plant nucleotide metabolism. Nucleotides are synthesized “de novo” from precursor molecules listed in the upper left box. The phosphorylation of nucleoside monophosphates (NMPs) via diphosphates (NDPs) generates nucleoside triphosphates (NTPs), which serve as building blocks for RNA synthesis and as precursors for the biosynthesis of the metabolites shown in the center (SAM, UDP-Glc, and NADH are given as examples). However, the NTPs, in particular ATP and GTP, are not only precursors for other metabolites, but are also essential stores of chemical energy in the phosphoanhydride bonds used in a multitude of energetic coupling reactions, as well as important donors of phosphate in kinase reactions (not shown). NDPs can be reduced to dNDPs, which after phosphorylation to dNTPs serve as precursors for DNA biosynthesis. RNA degradation in the cytosol releases nucleoside monophosphates, whereas nucleosides are produced during vacuolar RNA degradation. Adenosine and adenine are products of biochemical reactions involving SAM. Nonenzymatic decay (depurination) and enzymatic repair reactions result in nucleoside and nucleobase release from DNA. Nucleobases and nucleosides can be recycled to nucleotides in so-called salvage reactions. Plants are also capable of full nucleotide degradation via certain nucleosides and nucleobases releasing the nitrogen of the nucleobases as ammonia.