Abstract
The latest advances in the field exogenous application of RNA molecules in plants help to protect and modify them through RNA interference (RNAi).
Since its discovery more than 20 years ago, RNA interference (RNAi) has been extensively used in crop protection platforms. So far, RNAi approaches have been conventionally based on the use of transgenic plants expressing double-stranded RNAs (dsRNAs) against selected targets. However, the use of transgenes and genetically modified organisms (GMOs) has raised considerable scientific and public concerns. Hence emerged the need for alternative approaches that avoid the use of transgenes and resort instead to direct exogenous application of RNA molecules that have the potential to trigger RNAi. Here, we highlight the most important advances in this field, discussing the various methods of RNA delivery in plants against diverse targets such as plant genes, viruses, viroids, fungi, insects, mites, and nematodes. In addition, we examine the possible shortcomings of these methods, underline the critical parameters that have to be met for a desired outcome, and explore feasible possibilities to increase their efficiency and applicability, even against bacterial pathogens.
RNAi IN PLANTS
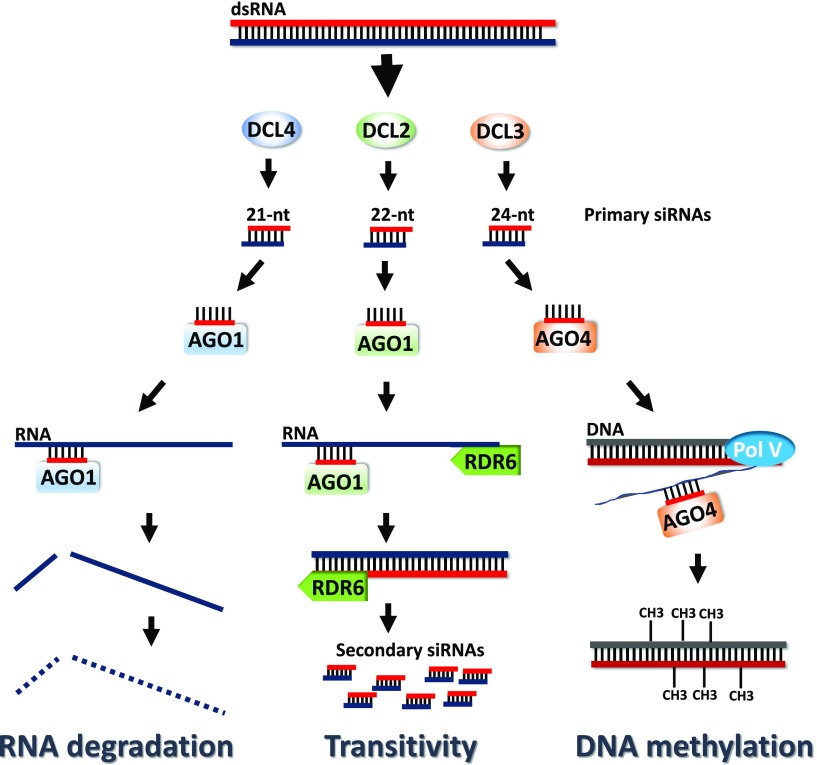
In plants, RNAi is triggered by dsRNA having variable sources of origin, ranging from viral replication intermediates, transcription of inverted repeats, stress-induced overlapping antisense transcripts, and RNA-DIRECTED RNA POLYMERASE (RDR) transcription of aberrant transcripts (Hamilton and Baulcombe, 1999; Mette et al., 1999; Borsani et al., 2005; Luo and Chen, 2007). Once present in the plant cell, dsRNAs are processed by DICER-LIKE (DCL) endonucleases into 21–24-nt short interfering RNAs (siRNAs; Fig. 1; Liu et al., 2009). The model plant Arabidopsis (Arabidopsis thaliana) contains four DCL paralogs. DCL2, DCL3, and DCL4 generate the 22-, 24-, and 21-nt siRNAs, respectively, whereas DCL1 recognizes genome-encoded imperfect hairpin RNAs resulting in the biogenesis of 21-nt/22-nt micro RNAs (miRNAs; Bologna and Voinnet, 2014; Borges and Martienssen, 2015). Plant siRNAs and miRNAs, collectively termed “small RNAs” (sRNAs; Ruiz-Ferrer and Voinnet, 2009), exhibit 3′ 2-nt overhangs and become stabilized though 3′ end methylation by HUA ENHANCER1 (Yu et al., 2005; Yang et al., 2006). After this modification, and depending on its 5′-terminal nucleotide, one of the two sRNA strands will be loaded onto an ARGONAUTE (AGO) protein (Kim, 2008). In general, 21-nt sRNAs with 5′-U are loaded on AGO1 and scan the cytoplasm for complementary transcripts for cleavage and degradation in a process termed “post-transcriptional gene silencing” (Hamilton and Baulcombe, 1999; Mi et al., 2008; Fig. 1). Twenty-two–nt sRNAs are also loaded on AGO1 but seemingly change AGO1 conformation and recruit RDR6 to the 3′ of the target, transcribing the target transcript into dsRNA and thus generating additional (secondary) siRNAs in a mechanism coined “transitive silencing” (Dalmay et al., 2000; Chen et al., 2010; Fig. 1). Finally, 24-nt sRNAs with 5′-A are incorporated on AGO4, recognize cognate DNA or its nascent transcript, and recruit DNA methyltransferases to methylate the cytosine residues of both DNA strands in a process termed “RNA-directed DNA methylation” (RdDM; Wassenegger et al., 1994; Chan et al., 2004; Fig. 1). Importantly, RNAi is not cell-autonomous in plants. Thus, once generated in a single cell, siRNAs are able to move through plasmodesmata to 10–15 neighboring cells while RNA molecules of a yet unknown nature move through the vasculature system to distant parts of the plant, a phenomenon called “systemic silencing” (Voinnet and Baulcombe, 1997; Palauqui and Vaucheret, 1998).
Figure 1.
DCL processing of exogenously applied dsRNA in plants. DCL4 generates 21-nt siRNAs that are loaded onto AGO1 and target complementary transcripts for cleavage. DCL2 generates 22-nt siRNAs that are also loaded onto AGO1 and recruit RDR6 to the target transcripts’ 3′ ends for the generation of secondary siRNAs. Finally, DCL3 generates 24-nt siRNAs that are loaded onto AGO4 and trigger de novo DNA methylation by hybridizing with nascent Pol V or Pol II transcripts. Whether DCL1, which is mainly involved in the miRNA pathway, also processes exogenously applied dsRNA, is not clear.
GMO-FREE RNAi IN PLANTS
In addition to mediating a broad range of developmental events, RNAi has great potential against invading pests and pathogens (Eamens et al., 2008; Martínez de Alba et al., 2013). So far, conventional RNAi applications have been largely based on the use of recombinant viruses (virus-induced gene silencing), Agrobacterium tumefaciens-mediated transiently expressed transgenes, and stably transformed transgenic plants that enable the production of dsRNA molecules against selected targets (host-induced gene silencing; Baulcombe, 2004, 2015). In 2017, the transgenic maize (Zea mays) SmartStax Pro, engineered to express dsRNA against corn rootworm (Diabrotica virgifera virgifera), was approved by the U.S. Environmental Protection Agency, the U.S. Food and Drug Administration, and the U.S. Department of Agriculture (https://www.epa.gov/newsreleases/epa-registers-innovative-tool-control-corn-rootworm). However, despite their demonstrated success, RNAi-based transgenic crops have not been commercialized as much as one might have expected. Transgenic plants and GMOs in general have met such severe criticism that their chances for widespread approval are gloomy, at the least. According to some estimates, it costs ∼140 million USD to bring a transgenic crop to commercialization (Rosa et al., 2018), and even when that happens, several anti-GMO responses follow. Taking these into consideration, and to tackle this issue, new GMO-free RNAi approaches need to be developed that will enable the activation of RNAi not through the use of recombinant viruses or transgenes but through the direct exogenous delivery of RNA molecules (dsRNAs and/or sRNAs) in plants. In this article, only GMO-free RNAi strategies involving exogenous application of RNA molecules directly in plants will be discussed; transgene-based host-induced gene silencing and virus-induced gene silencing approaches have been extensively reviewed elsewhere and will not be discussed here (Eamens et al., 2008; Prins et al., 2008; Nowara et al., 2010; Koch and Kogel, 2014; Baulcombe, 2015; Zotti and Smagghe, 2015; Joga et al., 2016; Cai et al., 2018a; Rosa et al., 2018; Zotti et al., 2018; Qi et al., 2019).
APPLYING RNA MOLECULES IN PLANTS TO TARGET ENDOGENES AND TRANSGENES
The first report wherein exogenous RNA application into plants triggered RNAi of a plant gene was described in a 2011 Monsanto patent; Nicotiana benthamiana plants pretreated with the surfactant Silwet L-77 (Momentive; https://www.momentive.com/en-us/categories/super-spreaders/silwet-l-77/) and sprayed (2.5 bar) with in vitro-transcribed 685-bp dsRNA and/or chemically synthesized 21-nt sRNAs targeting the endogenous phytoene desaturase mRNA displayed extensive phytoene desaturase RNAi (Sammons et al., 2011). (Note that because chemically synthesized sRNA oligonucleotide duplexes are not the biological outcome of DCL endonucleolytic processing and/or HUA ENHANCER1 methylation, they cannot be considered bona fide siRNAs, and thus will be collectively called hereafter as “sRNAs”). After this initial observation, several others followed using diverse methods of RNA application, succinctly mentioned below in chronological order. Hence, when Arabidopsis leaves were infiltrated with 21-nt sRNAs fused to a positively charged carrier peptide that combined a copolymer of His and Lys, (KH)9 (18 amino acids), RNAi of the yellow fluorescent protein (YFP) transgene and the chalcone synthase endogene was recorded (Numata et al., 2014). Subsequent studies demonstrated that RNA molecules can be absorbed by the roots and display biological activity throughout the plant. SHOOT MERISTEMLESS (STM) is a class-I knotted-like homeodomain protein expressed in the shoot apical meristem and required for shoot apical meristem formation, whereas WEREWOLF (WER) is an R2R3-type MyB-related transcription factor expressed in the root epidermal cells. When STM or WER dsRNA conjugated to cationic fluorescent nanoparticle was applied to Arabidopsis seedling roots for five consecutive days, expression of STM and WER was suppressed and resulted in phenotypic defects (Jiang et al., 2014). Moreover, when dsRNA targeting MOB1A and actin was applied to Arabidopsis and rice (Oryza sativa) roots, respectively, RNAi of the corresponding genes took place and normal root growth was perturbed (Li et al., 2015). These results suggest that irrigation-mediated RNA application is a possibility that needs to be further elaborated. In a subsequent study (Lau et al., 2014), instead of using in vitro-transcribed dsRNA, crude extracts of Escherichia coli HT115 (DE3; see “Applying RNA Molecules in Plants Against Viruses”) expressing a 430-bp dsRNA targeting MYB1 were mechanically inoculated into the hybrid orchid (Dendrobium hybrida) flower buds (Lau et al., 2015). MYB1 is expressed throughout flower bud development and is involved in the development of the conical cell shape of the epidermal cells of the flower labellum. Scanning electron microscopy of the adaxial epidermal cells revealed that application of MYB1 dsRNA changed the phenotype of floral cells, an outcome of great interest for floriculture biotechnology.
Plant cells contain a very tough cellulose-rich cell wall ranging from 0.1 µm to several micrometers in thickness, which poses a physical barrier to biomolecule delivery. To facilitate RNA delivery inside the plant cell, RNA molecules are usually conjugated to carrier compounds, as mentioned above (Jiang et al., 2014; Numata et al., 2014). Recently, DNA nanostructures, such as 3D tetrahedron, 1D hairpin tile, and 1D nanostring, were used to facilitate the delivery and biological action of 21-nt GFP sRNAs in infiltrated N. benthamiana leaves (Zhang et al., 2019). Instead of remaining in the mesophyllic apoplast, the nanostructure-conjugated sRNAs entered the symplast and silenced GFP expression. Interestingly, sRNAs tethered to 3D nanostructures exhibited both mRNA degradation and translational arrest of the GFP, whereas sRNAs attached to 1D nanostructures led mainly to translational arrest, although the reasons underlying this observation were not elucidated. It should be noted here that, although carrier compounds greatly facilitate RNA delivery, they are also quite expensive and/or difficult to synthesize. The use of carrier compounds seems to be dispensable when plant cell walls are mechanically damaged. Indeed, RNAi of a GFP transgene was efficiently initiated when 22-nt sRNAs or 720-bp dsRNAs (pure nucleic acids) were applied by high-pressure spraying (8 bar) or brush-mediated application on the leaf surface of N. benthamiana and Arabidopsis, respectively (Dalakouras et al., 2016; Dubrovina et al., 2019).
TRANSITIVITY AND SYSTEMIC SILENCING
Although 21-nt sRNAs (miRNAs and siRNAs) mediate RNA cleavage in plants, 22-nt sRNAs seemingly recruit RDR6 to the RNA target and are responsible for the amplification, transitive, and, likely, systemic spread of RNAi (Chen et al., 2010; Cuperus et al., 2010; McHale et al., 2013). Yet, it had been also suggested that recruitment of RDR6 to their target transcript is mediated not necessarily by 22-nt sRNAs, but by any-sized sRNA that contains an asymmetric bulge in its duplex structure (Manavella et al., 2012). To investigate how the sRNA size/structure affects the onset of local, transitive, and systemic RNAi in GFP-expressing N. benthamiana (Nb-GFP) plants, we applied, by high-pressure spraying, chemically synthesized 21-, 22-, and 24-nt GFP sRNAs either as perfect duplexes or as siRNAs containing an asymmetric bulge (Dalakouras et al., 2016). Ultraviolet light examination of sprayed plants revealed that although all tested sRNAs triggered local RNAi to variable degrees, only 22-nt sRNAs (either as perfect duplexes or containing an asymmetric bulge) also triggered systemic silencing (Fig. 2, left). DCL2, responsible for the biogenesis of 22-nt siRNAs, is required for transitivity (Mlotshwa et al., 2008; Parent et al., 2015). Moreover, 22-nt miRNAs recruit RDR6 and trigger transitivity (Chen et al., 2010; Cuperus et al., 2010; McHale et al., 2013). Thus, it is reasonable to assume that any sRNA (siRNA or miRNA or chemically synthesized oligonucleotide) having a size of 22 nt can recruit RDR6 to its target and lead to the biogenesis of secondary siRNAs. Upon transitivity, the siRNA population surpasses a certain threshold required for the onset of systemic silencing (Kalantidis et al., 2006, 2008). Thus, transitivity is seemingly connected to generation of systemic silencing signals in the source tissues. Yet, reception of systemic silencing in the sink tissues requires RDR6 processing of the target (Schwach et al., 2005). A growing body of recent evidence has suggested that in contrast to intron-containing genes, intronless genes are much more susceptible to RDR6 processivity and thus transitivity and systemic silencing (Christie and Carroll, 2011; Christie et al., 2011; Dadami et al., 2013, 2014). Taking these observations into consideration, the chances of systemic RNAi upon exogenous RNA application may significantly increase when then the trigger is 22-nt sRNA and the target gene is intronless (see “Outstanding Questions”).
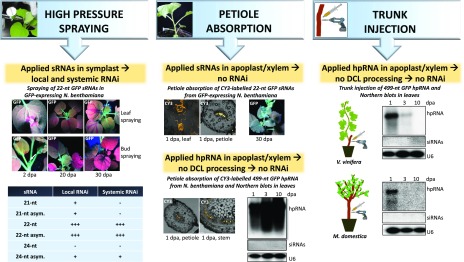
Figure 2.
Methods for RNA delivery to plants. Left, High-pressure spraying of chemically synthesized GFP sRNAs in Nb-GFP allows the delivery of sRNAs into the symplast and initiation of RNAi. Only the 22-nt GFP sRNAs, with or without an asymmetric bulge, are able to trigger efficient local (2 dpa) and systemic (20 dpa) RNAi (ultraviolet examination). Notably, targeting the bud instead of the leaf results in more pronounced and widespread RNAi. Middle, Petiole absorption of chemically synthesized sRNAs and in vitro-transcribed GFP hpRNAs results in the localization of applied RNAs in the apoplast and xylem (confocal microscopy of CY3-labeled RNAs). Thus, the applied 22-nt GFP sRNAs fail to induce RNAi in Nb-GFP (ultraviolet examination) whereas the applied GFP hpRNA is not processed by DCLs into GFP siRNAs (northern blot analysis). Right, Trunk injection of in vitro-transcribed GFP hpRNA into apple and grapevine results in its localization in the apoplast and xylem. Thus, similar to petiole absorption, no DCL processing takes place (northern blot analysis). Image adapted from Dalakouras et al. (2016, 2018).
SYMPLASTIC AND APOPLASTIC DELIVERY OF RNA
It needs to be noted here that depending on the method of RNA application, the efficiency of RNAi fluctuates. When chemically synthesized 22-nt sRNAs were applied in Nb-GFP upon high-pressure spraying, they triggered local and systemic RNAi; yet, when they were applied by petiole absorption, they failed to do so (Dalakouras et al., 2018). Confocal microscopy revealed that the petiole-absorbed sRNA was transported through the xylem and was restricted to the apoplast (Fig. 2, middle; Dalakouras et al., 2018). Similarly, a 499-nt GFP hairpin RNA (hpRNA) applied through petiole absorption and/or trunk injection in grapevine (Vitis vinifera) and apple (Malus domestica) trees was also confined to the xylem and apoplast, and no RNAi was observed (Fig. 2, middle and right). In summary, when the target of exogenously applied RNA is an intracellular transcript such as plant mRNA or viral/viroid RNA (Fig. 3, left), then the high-pressure method ensures efficient RNA delivery into plant cells leading to the onset of, at least, local RNAi. In contrast, RNA applied through petiole absorption and trunk injection is retained in the xylem and apoplast and does not trigger RNAi. However, this is not necessarily a drawback. When dsRNAs are applied to plants with the aim of targeting some insects or fungi (Fig. 3, right), it may be of advantage to deliver nonprocessed (by plant DCLs) dsRNAs. Should the plant-applied dsRNAs avoid plant DCL processing, they will be taken up intact by the insects or fungi and be processed therein by the pathogen/pest DICER proteins; the resulting siRNAs will conceivably exhibit greater biological activity against insect or fungal genes. To achieve this, recent approaches rely on the expression of dsRNA transgenes in chloroplasts (Bally et al., 2016, 2018). Chloroplasts lack DCLs and thus the chloroplast-expressed dsRNAs remain intact for pathogen/pest uptake. Our own observations suggest that dsRNA application through petiole uptake and/or trunk injection could serve as a GMO-alternative to such transplastomic plants because they both maintain dsRNA intact.
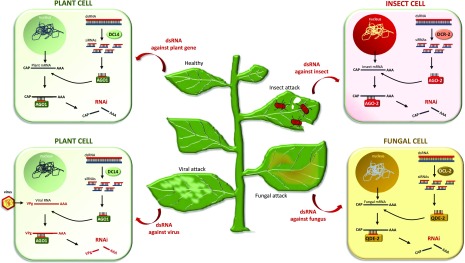
Figure 3.
Exogenous application of RNA molecules into plants against various targets, such as endogenous plant genes, viruses, insects, and fungi. In the first two cases, RNAi should take place inside the plant cell. Thus, the most suitable application method is high-pressure spraying, which allows symplastic RNA delivery. In contrast, in the cases of insects and fungi, RNAi takes place inside the insect and fungal cells, which thus need to uptake intact dsRNA (unprocessed by the plant DCLs) to achieve efficient RNAi. Hence, in these cases, trunk injection, petiole absorption, and/or low-pressure spraying (wherein RNA stays on the leaf surface) are the most suitable methods, because these methods do not result in symplastic RNA delivery. Importantly, although trunk injection and/or petiole absorption is the ideal method for xylem-feeding and/or chewing insects, the spraying method would be more suitable for phloem-feeding insects (e.g. aphids).
THE ISSUE OF EPIGENETIC CHANGES
So far, all reports on exogenous applications of dsRNAs in plants aimed to trigger degradation of a given mRNA. However, once present in the plant cell, the applied dsRNAs may be processed not only by DCL4/DCL2 into 21-/22-nt siRNAs but also by DCL3 into 24-nt siRNAs, given the DCL colocalization (Pontes et al., 2013; Pumplin et al., 2016). Thus, besides mRNA degradation in the cytoplasm, the applied dsRNAs may also trigger RdDM of the cognate coding region in the nucleus (Dubrovina et al., 2019; Dubrovina and Kiselev, 2019). Thus, dsRNA-treated plants considered on the one hand GMO-free, could, on the other hand, be epigenetically modified. Moreover, exogenous application of dsRNA targeting a promoter sequence may trigger not only RdDM but also histone modifications that will eventually result in transcriptional gene silencing (Wassenegger, 2005). If RdDM takes place in the dsRNA-treated plants, DOMAINS REARRANGED METHYLTRANSFERASE will mediate the de novo methylation of the cognate DNA sequence (promoter or coding region) at any sequence context: CG, CHG, and CHH (Pélissier et al., 1999; Cao and Jacobsen, 2002; Aufsatz et al., 2004). In the progeny of treated plants, CG and, to a lesser extent, CHG methylation can be maintained by METHYLTRANSFERASE1 and CHROMOMETHYLASE3, respectively (Lindroth et al., 2001; Aufsatz et al., 2004). Although CG/CHG methylation of a coding region does not seem to negatively affect transcription in plants, CG/CHG methylation of promoter sequences reinforces the heterochromatic state, and thus, transcriptional gene silencing can be transgenerationally maintained (Wang et al., 2015; Wakasa et al., 2018). Whether such scenario holds remains to be validated (see “Outstanding Questions”).
APPLYING RNA MOLECULES IN PLANTS AGAINST VIRUSES
It has been suggested that RNAi in plants evolved as an antiviral defense mechanism (Baulcombe, 2004). Indeed, most plant viruses contain single-stranded genomes that replicate through dsRNA intermediates. The dsRNA is processed into virus-derived siRNAs resulting in degradation of any homologous RNA, including the single-stranded virus RNA genomes. To counter the host’s defense, viruses encode RNA silencing suppressors, most of which sequester and/or inhibit the function of the siRNAs (Silhavy and Burgyán, 2004, 2013). However, the cellular pre-existence of dsRNAs/siRNAs designed to target the virus already before it manages to replicate and generate RNA silencing suppressors is a well-established antiviral crop protection strategy. The GMO approach wherein transgenic plants express dsRNAs against viral proteins has been well documented with very satisfactory results (Prins et al., 2008; Khalid et al., 2017; Pooggin, 2017). Focusing here only on the nontransgenic approaches, Tenllado and coworkers were the first to demonstrate that exogenous application of dsRNA molecules in plants renders them resistant to viral infections. When pepper mild mottle virus, tobacco etch virus, and alfalfa mosaic virus were mechanically coinoculated in N. benthamiana leaves with in vitro-transcribed 977-bp, 1,483-bp, and 1,124-bp dsRNAs targeting the pepper mild mottle virus replicase, tobacco etch virus helper component, and alfalfa mosaic virus RNA3, respectively, viral infections were attenuated (Tenllado and Díaz-Ruíz, 2001). The authors applied crude extract from E. coli HT115 (DE3) expressing the corresponding dsRNA fragments by lysing cell pellets with a French press and documented similar resistance phenotypes (Tenllado et al., 2003). Using bacterial expression systems, dsRNA expression can easily be scaled-up and still remain cost-effective. With an average dsRNA yield of 4 μg/mL of bacterial culture, large fermenters would most possibly meet the huge agricultural demands (Tenllado et al., 2004). Besides HT115 (DE3), alternative strains such as M-JM109lacY have been used with equally satisfactory results. Mechanical inoculation of RNA extract from E. coli M-JM109lacY expressing 480-bp dsRNA that target the tobacco mosaic virus (TMV) coat protein (CP) and RNA extract from E. coli HT115 (DE3) expressing 480-bp dsRNA targeting the TMV movement protein both resulted in viral resistance in tobacco (Nicotiana tabacum; Yin et al., 2009, 2010). Recently, an alternative system for high-quality long dsRNA production was developed, based on Pseudomonas syringae harboring components of the bacteriophage phi6, wherein dsRNA generation is based not on DNA transcription but on RNA transcription by an RNA-dependent RNA polymerase (Niehl et al., 2018). Compared to E. coli-expressed dsRNA, which may result in relatively low yields of fully duplexed dsRNA, the P. syringae system seems advantageous because the RNA-dependent RNA polymerase of phage phi6 converts single-stranded RNA templates into dsRNA with high processivity using a de novo, primer-independent initiation mechanism.
Using the aforementioned methods, viral resistance has been achieved in a plethora of other cases as listed here: in maize, upon spraying of crude extract from E. coli HT115 (DE3) expressing dsRNA targeting the sugarcane mosaic virus CP (Gan et al., 2010); in papaya (Carica papaya), upon mechanical inoculation of crude extract from E. coli M-JM109lacY expressing 279-bp dsRNA targeting the papaya ringspot virus CP (Shen et al., 2014); in pea (Pisum sativum), upon biolistic delivery of in vitro-transcribed 500-bp dsRNA targeting the pea seed-borne mosaic virus CP (Safarova et al., 2014); in orchid (Brassolaeliocattleya hybrida), upon mechanical inoculation of crude extract from E. coli HT115 (DE3) expressing dsRNA targeting the cymbidium mosaic virus CP (Lau et al., 2014); in tobacco, upon mechanical inoculation of in vitro-transcribed 237-bp dsRNA targeting the TMV p126 (Konakalla et al., 2016); in cucurbits, upon mechanical inoculation of in vitro-transcribed 588-bp dsRNA targeting the zucchini yellow mosaic virus helper component proteinase (Kaldis et al., 2018); and in N. benthamiana, upon spraying of RNA extracts from P. syringae LM2691 expressing dsRNAs targeting a 2,611-bp region of TMV replicase and movement protein (Niehl et al., 2018).
A major issue concerning the aforementioned approaches is that dsRNA application offers a short antiviral protection window (usually 5–10 d), because dsRNA eventually is degraded. Thus, dsRNA would need to be supplied afresh in frequent intervals for lifelong crop protection. To increase the dsRNA stability and thus prolong antiviral protection, Mitter and coworkers bound dsRNA to layered double hydroxide clay nanosheets having an average particle size of 80–300 nm (BioClay; Mitter et al., 2017). The dsRNA bound to BioClay was significantly protected from nucleases, while the dsRNA/BioClay complex did not wash off, even after rigorous rinsing. On the leaf surface, atmospheric CO2 and moisture resulted in a gradual breakdown of BioClay into a biocompatible residue, releasing the dsRNA in the plant cell either by passive diffusion or active transport. A 330-bp dsRNA targeting the CMV CP and bound to BioClay could be detected even 30 d post-application (dpa) and upon single application in tobacco resulted in CMV protection for at least 20 d (Mitter et al., 2017). Similar results were recently obtained, when spraying of 461-bp dsRNA bound to BioClay and targeting the bean common mosaic virus CP, protected N. benthamiana and cowpea (Vigna unguiculata) plants against bean common mosaic virus infection (Worrall et al., 2019).
APPLYING RNA MOLECULES IN PLANTS AGAINST VIROIDS
Viroids are infectious single-stranded RNA pathogens that, unlike viruses, are nonencapsidated and noncoding but, similar to viruses, trigger the host RNAi mechanism (Tabler and Tsagris, 2004; Tsagris et al., 2008; Flores et al., 2014, 2017). Single-stranded circular RNA viroids exhibit significant intramolecular folding and resemble dsRNA molecules and as such are processed by plant DCLs into siRNAs. Initial reports suggested that viroids are resistant to siRNA-mediated degradation due to their extensive secondary structure (Itaya et al., 2007). Yet, subsequent studies demonstrated that transgenic plants expressing viroid dsRNAs were viroid-resistant (Schwind et al., 2009). Hence, despite their secondary structure, and similar to viruses, viroids can most likely be targeted for sRNA-mediated degradation (Dalakouras et al., 2015; Flores et al., 2017). Returning to the nontransgenic approaches, to the best of our knowledge there is only one study where exogenous RNA was applied in plants for antiviroid resistance, wherein Carbonell and coworkers applied dsRNA in plants against both nuclear-replicating Pospiviroidae and chloroplast-replicating Avsunviroidae members. In tomato (Solanum lycopersicum), gynura (Gynura aurantiaca), and chrysanthemum (Dendranthema grandiflora), mechanical inoculation of in vitro-transcribed 353-, 364-, and 399-bp dsRNA targeting regions of Potato spindle tuber viroid, Citrus exocortis viroid, and Chrysanthemum chlorotic mottle viroid, respectively, resulted in significant inhibition of infection, at least for up to 30 dpa (Carbonell et al., 2008). Interestingly, the effect was temperature-dependent, reminiscent of previous reports on the temperature-dependency of the general RNAi mechanism in plants (Kalantidis et al., 2002; Szittya et al., 2003). Taking these observations into consideration, exogenous application of RNA in plants for any applications should be performed in a temperature regime of 20οC to 30οC, since the efficiency of the RNAi machinery is compromised outside this temperature window.
APPLYING RNAi MOLECULES IN PLANTS AGAINST FUNGI
With the notable exceptions of Saccharomyces cerevisiae and Ustilago maydis, most fungi, like the rest of eukaryotes, contain several DICER and AGO genes and thus display active RNAi mechanisms. Transgenic plants expressing dsRNAs against fungal genes is a very promising antifungal approach (Koch and Kogel, 2014; Baulcombe, 2015) but will not be addressed here. Koch and coworkers demonstrated that spraying of barley (Hordeum vulgare) with in vitro-transcribed 791-bp dsRNA simultaneously targeting three Fusarium graminearum ergosterol biosynthesis genes (CYP51A, CYP51B, and CYP51C) strongly inhibited fungal growth (Koch et al., 2016). Interestingly, the sprayed dsRNA was poorly processed in barley into siRNAs, and the effect on fungal growth was due to the uptaken dsRNA rather than the barley-produced siRNAs, because upon dsRNA spraying no RNAi was observed in a dcl1 F. graminearum mutant. Spraying of in vitro-produced siRNAs also inhibited fungal growth but to lesser extent than dsRNA did. Surprisingly, dsRNA seemed to be more mobile throughout the barley than the smaller siRNAs, but the reasons underlying this phenomenon are unclear. It is also unclear how the dsRNA that first reached the apoplast was transported to the symplast (Koch et al., 2016). In another study, foliar application in Brassica napus of in vitro-transcribed dsRNA targeting various fungal genes conferred plant protection against Sclerotinia sclerotium and Botrytis cinerea (McLoughlin et al., 2018). More recently, spraying of in vitro-transcribed dsRNA targeting the myosine5 of Fusarium asiaticum in wounded wheat (Triticum aestivum) coleoptiles resulted in reduced fungal virulence (Song et al., 2018). Yet, the RNAi effect lasted for only 9 h, unless the dsRNA was continuously supplied. Interestingly, despite the presence of RDR genes in F. asiaticum, sRNA deep sequencing revealed that no secondary siRNAs were generated, suggesting that fungal myosine5 mRNA could not be processed by RDR (Song et al., 2018). Thus, in contrast to plants where RNAi is maintained through an RDR-mediated self-amplification loop, this is likely not happening in fungi.
Recent advances from the Hailing Jin laboratory have broadened our knowledge of the mechanistic details of RNA transport phenomena between plants and fungi. Hence, it was discovered that the aggressive fungal pathogen B. cinerea is able not only to deliver siRNAs into host plant cells to suppress host immunity genes, but also uptake exogenously applied dsRNAs and siRNAs that inhibit its growth, providing a typical case of bidirectional trans-kingdom RNAi (Weiberg et al., 2013; Wang et al., 2016). More specifically, when in vitro-transcribed dsRNA or siRNAs targeting the Bc-DCL1 and Bc-DCL2 genes were applied on the surface of fruits (tomato [S. lycopersicum ‘Roma’]; strawberry [Fragaria × ananassa]; and fox grape [Vitis labrusca ‘Concord’]), vegetables (iceberg lettuce [Lactuca sativa]; and onion [Allium cepa]), and flowers (rose [Rosa hybrid]), they significantly inhibited gray mold disease (Wang et al., 2016). These findings illustrated that exogenous RNA application can be used to control diseases in post-harvest fruits and vegetables. Whereas the movement of siRNAs through plasmodesmata to neighboring cells and through the vasculature system to distant parts of the plant is well established, it was unknown until recently how siRNAs/dsRNAs travel across the boundaries between organisms of different taxonomic kingdom, e.g. from plants to fungi and/or vice versa. To investigate how RNA molecules move from plants into interacting fungal cells, Hailing Jin and coworkers demonstrated that Arabidopsis secretes exosome-like extracellular vesicles to deliver RNA molecules into B. cinerea (Cai et al., 2018b). It is reasonable to assume that such observations reflect a more generalized mechanism of exosome involvement in trans-kingdom RNA transport, and if so, exosomes are likely to be employed in the future in the development of antifungal RNA delivery methods.
APPLYING RNAi MOLECULES IN PLANTS AGAINST INSECTS
Triggering RNAi in insects upon exogenous application of dsRNA in plants is a challenging task. Subsequent to the uptake by chewing/sucking insects, the dsRNA must survive the salivary nucleases in the midgut and/or hemolymph, which threaten to quickly degrade it. Next, the dsRNA must be taken up from the epithelial cells through either the endocytic pathway or the transmembrane Sid-1 channel protein-mediated pathway and processed by Dicer-2 into siRNAs, which will be loaded onto Ago-2 and trigger local RNAi (Fig. 3). To be efficiently established throughout the insect, the RNAi molecules (dsRNAs or siRNAs) need to systemically spread to neighboring and to distant cells. In plants and the nematode Caenorhabditis elegans, systemic spread of RNAi is associated with RNAi amplification by RDRs, proteins that are not present in insects. Yet, despite all these barriers, a proof-of-concept article demonstrated that western corn rootworm ingestion of vacuolar ATPase dsRNA through artificial diet or upon feeding on dsRNA-expressing transgenic maize plants resulted in significant larval mortality, suggesting that widespread RNAi in insects can be established. Indeed, coleopterans seem to exhibit systemic RNAi spread, although the enzymes involved have not been identified yet (Vélez and Fishilevich, 2018).
Induction of RNAi in insects through the use of methods such as dsRNA injection, dsRNA-expressing transgenic plants, dsRNA-containing artificial diet, or dsRNA-coating of leaf discs has been thoroughly reviewed elsewhere (Zotti and Smagghe, 2015; Joga et al., 2016; San Miguel and Scott, 2016; Bally et al., 2018; Vélez and Fishilevich, 2018; Zotti et al., 2018). Exogenous application of dsRNA directly in plants against insects was first demonstrated by Hunter and coworkers, who injected the trunk and/or drenched the roots of citrus and grapevine trees with dsRNA targeting the Arg kinase of two psyllids (Diaphorina citri and Bactericera cockerelli) and the sharpshooter Homalodisca vitripennis. Interestingly, the introduced dsRNA was detected in 2.5-m tall 6-year–old Mexican limes (Citrus aurantifolia) even after 7 weeks post-single application (2-g dsRNA diluted in 15 L of water). After this proof-of-concept report, several others followed, exhibiting significant degrees of pest management upon exogenous application of in vitro-transcribed/synthesized RNAs in plants: in Brassica oleracea, upon spraying of 5′-PEG 21-nt siRNAs targeting the diamondback moth Plutella xylostella acetylcholine esterase genes AChE1 and AChE2; in tomato, upon application of dsRNA targeting the D. virgifera vacuolar ATPase (Ivashuta et al., 2015); in rice, upon root absorption of dsRNA targeting the brown planthopper Nilaparyata lugens P450 (Li et al., 2015); in maize, upon root absorption of dsRNA targeting the Ostrinia furnacalis KTI (Li et al., 2015); in potato (Solanum tuberosum), upon spraying of dsRNA targeting the Colorado potato beetle Leptinosa decemlineata actin; in tomato, upon petiole uptake of dsRNA targeting the tomato leafminer Tuta absoluta vacuolar ATPase (Camargo et al., 2016), and upon root absorption of dsRNA targeting T. absoluta ryanodine, acetylcholinesterase, and nicotinic acetylcholine alpha6 (Majidiani et al., 2019). In general, the orders Coleoptera, Lepidoptera, and Hemiptera include key pests of crops. Coleopterans are the most susceptible to RNAi, whereas lepidopterans and hemipterans seem recalcitrant to RNAi, most likely because lepidopterans restrict the uptaken dsRNA to endocytic compartments, and hemipterans inject nucleases into the plant tissue before feeding (Lomate and Bonning, 2016; Shukla et al., 2016). Of note, and to improve dsRNA stability, uptake, and overall RNAi response, various insecticidal dsRNA formulations have been explored, such as liposomes, chitosan nanoparticles, cationic core-shell nanoparticles, and guanylated polymers (Joga et al., 2016; Vélez and Fishilevich, 2018).
APPLYING RNA MOLECULES IN PLANTS AGAINST MITES
Mites belong to the subphylum Chelicerata, a subgroup in the phylum Arthropoda to which insects also belong. Mite genomes encode for most known RNAi enzymes, including two DICERs, seven AGOs, and, importantly, five RDRs (Grbić et al., 2011), suggesting that amplification of RNAi and systemic RNAi spread in mites may occur. Indeed, in the citrus red mite Panonychus citri, RNAi spread from the gut to more distant tissues such as cuticle, hemolymph, and prothoracic glands (Li et al., 2017). Similar to insects, mites have been targeted for RNAi by various methods, such as feeding on leaves floating on dsRNA solution, dsRNA-expressing transgenic plants, and dsRNA-containing artificial diet, as discussed elsewhere (Suzuki et al., 2017; Niu et al., 2018). To the best of our knowledge, the only study wherein mites took up dsRNA that was exogenously applied in plants involved mechanical inoculation of tomato leaves with in vitro-transcribed dsRNA (targeting the otherwise irrelevant zucchini yellow mosaic virus helper component proteinase) and the concomitant detection by stem loop reverse transcription PCR of the corresponding siRNAs in the two-spotted spider mite Tetranychus urticae at 3 and 10 dpa (Gogoi et al., 2017). However, in that case it was not possible to determine whether the mite absorbed the applied dsRNA or the plant-produced siRNAs. In addition, no conclusions could be drawn concerning the RNAi action of the detected siRNAs, because the applied dsRNA had no mite target. In another study where T. urticae was fed on Arabidopsis leaves coated with in vitro-transcribed dsRNA targeting the vacuolar ATPase, the mite exhibited visible dark body phenotype but lethality was not observed (Suzuki et al., 2017). Interestingly, to identify the most suitable RNAi target genes for mite mortality, two independent studies suggested that coatamer I genes and genes involved in the biosynthesis and action of juvenile hormone and action of ecdysteroids were the best candidates (Kwon et al., 2016; Yoon et al., 2018) and should be thus taken into consideration for future RNA applications.
APPLYING RNA MOLECULES IN PLANTS AGAINST NEMATODES
RNAi was discovered in the model nematode C. elegans (Fire et al., 1998). In agriculture, root-knot nematodes cause significant crop losses in several plant species worldwide. Transgenic Arabidopsis expressing 16D10 dsRNA against Meloidogyne incognita, Meloidogyne javanica, Meloidogyne arenaria, and Meloidogyne hapla exhibited significant reductions in the number of nematode eggs per gram of root (Huang et al., 2006). So far, no reports on exogenous RNA application in plants against nematodes are available. Arguably, the challenge here would be to deliver RNA molecules to the root tissues. Trunk injection would deliver RNA mainly to the xylem (Dalakouras et al., 2018) and thus only to the upper/aerial part of the plant. Alternatively, RNA molecules can be directly absorbed by the roots (Jiang et al., 2014; Li et al., 2015). It is thus reasonable to assume that RNA molecules conjugated to compounds that would protect them against degradation in the aggressive soil environment could be delivered through irrigation as a nematode protection strategy.
PERSPECTIVES FOR BACTERIAL MANAGEMENT
Plant pathogenic bacteria may cause devastating diseases and huge crop losses. Although RNAi is absent in bacteria, similar RNA-based regulation mechanisms do exist in which ∼100-nt cis and trans antisense transcripts repress translation and lead to transcript decay (Good and Stach, 2011). Typically, antisense RNAs (asRNAs) that are cis-encoded display high degrees of complementarity with the target mRNA, whereas asRNAs that are trans-encoded exhibit limited complementarity with the target mRNA and require Hfq chaperones to facilitate binding to the target mRNA. So far, exogenous application in plants of synthetic asRNAs designed to combat bacterial pathogens has not been tested. In contrast to dsRNAs applied in RNAi approaches, asRNAs are single-stranded and thus more susceptible to plant nucleases. To tackle this problem, exogenously applied asRNAs based on peptide nucleic acids and/or phosphorothioate morpholino oligomers could be used (Good and Stach, 2011). Yet, the biggest challenge upon exogenous application of synthetic asRNAs would be the intracellular delivery across the stringent bacterial cell barriers, because nucleobase oligomers are too large for uptake by simple diffusion. To this end, conjugating the peptide nucleic acid or phosphorothioate morpholino oligomers to cell cationic entry peptides would greatly facilitate their intracellular delivery (Mellbye et al., 2009). These being said, exogenously applied antibacterial asRNAs could be used with considerable chances of success, particularly in the case of xylem-restricted bacteria such as Xylella fastidiosa, Clavibacter xyli, and Pseudomonas syzygii, especially when taking into account that delivery of RNA with robust methods, such as trunk injection or petiole absorption, restricts the presence of the applied RNA to the xylem (Dalakouras et al., 2018). Besides the aforementioned direct management of bacterial pathogens through exogenous application of synthetic asRNAs, indirect RNAi-based strategies could be applied. For example, although RNAi cannot be used directly against X. fastidiosa, it can nevertheless be employed against its xylem sap-feeding vectors, mainly sharpshooters and spittlebugs (Rosa et al., 2010; Overall and Rebek, 2015). Trunk injected-dsRNAs designed to target essential insect mRNAs will remain unprocessed in the xylem of the woody plant, and provided that they are taken up by the xylem-feeding insect, they will be processed into siRNAs that that will lead to RNAi-mediated insect lethality, ideally minimizing the spread of the devastating pathogen (see “Outstanding Questions”).
CONCLUDING REMARKS
For field-scale management of pests and pathogens, metric-ton levels of dsRNAs would be required. A rough estimation suggests 10 g of dsRNA per hectare, although this amount may vary depending on the target sensitivity to RNAi and its capacity for systemic RNAi (Zotti et al., 2018). Conceivably, such huge needs could not be merely met by in vitro dsRNA transcription systems, which exhibit a minimum cost of 100 USD per 1 g of dsRNA. To this end, several industrial companies enable low-cost (almost 2 USD per 1 g of dsRNA), large-scale manufacturing of dsRNA (Zotti et al., 2018). According to “RNAagri” proprietary technology (https://www.rnagri.com/), bacteria and yeast are engineered to produce the desired dsRNA and a capsid protein that offers protection against nucleases. As the bacteria multiply in the fermentators, the dsRNA:protein complex is self-assembled and accumulates in huge quantities that can be isolated with conventional low-cost methods. The final product is environmentally stable and ready-to-use. Field-scale trunk injection of such huge amounts of dsRNAs could be performed by commercially available trunk drilling equipment such as the one developed by “Arborjet” (https://arborjet.com/arborjet-advantage/). Such advances have facilitated the development of commercial products that are soon to emerge in the market, such as “BioDirect,” which is designed for insect, virus, and weed control and which has already passed the phase I or II of the research and development process (Zotti et al., 2018).
In summary, exogenous application of RNA molecules with the potential to trigger RNAi is a very powerful tool in modern crop protection and improvement platforms, considering the political and public pressure for sustainable solutions to current agricultural problems. According to the the 40th Annual Meeting of The Toxicology Forum, the currently available evindence suggests that the presence of RNAi molecules in human diet pose no threat to humans (Sherman et al., 2015). Moreover, compared to conventional disease management strategies, exogenous RNAi promises significantly lower off-target effects, because its activity can be narrowed down to a window of a few nucleotides’ complementarity with its target. With the rapidly accumulating sequence datasets, nonconserved regions can be selected as targets to exclude as much as possible off-target effects. To the same end, applying unique sRNAs, rather than long dsRNAs, which are processed in a diverse population of siRNAs, would also greatly decrease off-targets. Arguably, the lack of genome data for any existing organisms near the site of exogenous RNA application undermines the proper ecological risk of RNAi technology in the agroecosystem. Nevertheless, the chance to silence nonintended targets is very low and far beyond any other available crop protection strategy has yet to offer. It cannot be excluded that certain pathogens may display gene mutations rendering them resistant to RNAi sprays. In cases of monogenic resistance, alternating ∼200-bp regions of the target sequence could tackle this issue, whereas for polygenic resistance cases, using chimeric RNAs or a mixture of different RNAs could offer a solution, underpinning the overall flexibility of the RNA-based approaches. Importantly, it was recently demonstrated that the RNAi efficiency in plants can be enhanced by the addition of chemical enhancers such as Sortin1, Isoxazolone and [5-(3,4-dichlorophenyl)furan-2-yl]-piperidine-1-ylmethanethione (Jay et al., 2019). In the near future, it is reasonable to assume that optimization of RNA production, delivery, and stability methods may pave the way for the exogenous application of diverse RNA molecules not necessarily triggering only RNAi. Indicatively, such molecules could include: capped and polyadenylated mRNAs when accumulation of a protein is desired; miRNA decoys/sponges for sequestering of a given miRNA and thus upregulating its endogenous target; and even clustered regularly interspaced short palindromic repeats components (CRISPR; CRISPR associated protein 9 mRNA and single guide RNAs ) for the induction of editing events in a GMO-free manner (see “Outstanding Questions”).
Footnotes
This work was supported by the European Union’s Horizon 2020 Research and Innovation Programme (Marie Skłodowska-Curie grant agreement no. 793186 RNASTIP to A.D. and K.P.).
Articles can be viewed without a subscription.
References
- Aufsatz W, Mette MF, Matzke AJ, Matzke M (2004) The role of MET1 in RNA-directed de novo and maintenance methylation of CG dinucleotides. Plant Mol Biol 54: 793–804 [DOI] [PubMed] [Google Scholar]
- Bally J, McIntyre GJ, Doran RL, Lee K, Perez A, Jung H, Naim F, Larrinua IM, Narva KE, Waterhouse PM (2016) In-plant protection against Helicoverpa armigera by production of long hpRNA in chloroplasts. Front Plant Sci 7: 1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bally J, Fishilevich E, Bowling AJ, Pence HE, Narva KE, Waterhouse PM (2018) Improved insect-proofing: Expressing double-stranded RNA in chloroplasts. Pest Manag Sci 74: 1751–1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulcombe D. (2004) RNA silencing in plants. Nature 431: 356–363 [DOI] [PubMed] [Google Scholar]
- Baulcombe DC. (2015) VIGS, HIGS and FIGS: Small RNA silencing in the interactions of viruses or filamentous organisms with their plant hosts. Curr Opin Plant Biol 26: 141–146 [DOI] [PubMed] [Google Scholar]
- Bologna NG, Voinnet O (2014) The diversity, biogenesis, and activities of endogenous silencing small RNAs in Arabidopsis. Annu Rev Plant Biol 65: 473–503 [DOI] [PubMed] [Google Scholar]
- Borges F, Martienssen RA (2015) The expanding world of small RNAs in plants. Nat Rev Mol Cell Biol 16: 727–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsani O, Zhu J, Verslues PE, Sunkar R, Zhu JK (2005) Endogenous siRNAs derived from a pair of natural cis-antisense transcripts regulate salt tolerance in Arabidopsis. Cell 123: 1279–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, He B, Kogel KH, Jin H (2018a) Cross-kingdom RNA trafficking and environmental RNAi—nature’s blueprint for modern crop protection strategies. Curr Opin Microbiol 46: 58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q, Qiao L, Wang M, He B, Lin FM, Palmquist J, Huang SD, Jin H (2018b) Plants send small RNAs in extracellular vesicles to fungal pathogen to silence virulence genes. Science 360: 1126–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo RA, Barbosa GO, Possignolo IP, Peres LE, Lam E, Lima JE, Figueira A, Marques-Souza H (2016) RNA interference as a gene silencing tool to control Tuta absoluta in tomato (Solanum lycopersicum). PeerJ 4: e2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Jacobsen SE (2002) Role of the Arabidopsis DRM methyltransferases in de novo DNA methylation and gene silencing. Curr Biol 12: 1138–1144 [DOI] [PubMed] [Google Scholar]
- Carbonell A, Martínez de Alba AE, Flores R, Gago S (2008) Double-stranded RNA interferes in a sequence-specific manner with the infection of representative members of the two viroid families. Virology 371: 44–53 [DOI] [PubMed] [Google Scholar]
- Chan SW, Zilberman D, Xie Z, Johansen LK, Carrington JC, Jacobsen SE (2004) RNA silencing genes control de novo DNA methylation. Science 303: 1336. [DOI] [PubMed] [Google Scholar]
- Chen HM, Chen LT, Patel K, Li YH, Baulcombe DC, Wu SH (2010) 22-Nucleotide RNAs trigger secondary siRNA biogenesis in plants. Proc Natl Acad Sci USA 107: 15269–15274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie M, Carroll BJ (2011) SERRATE is required for intron suppression of RNA silencing in Arabidopsis. Plant Signal Behav 6: 2035–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie M, Croft LJ, Carroll BJ (2011) Intron splicing suppresses RNA silencing in Arabidopsis. Plant J 68: 159–167 [DOI] [PubMed] [Google Scholar]
- Cuperus JT, Carbonell A, Fahlgren N, Garcia-Ruiz H, Burke RT, Takeda A, Sullivan CM, Gilbert SD, Montgomery TA, Carrington JC (2010) Unique functionality of 22-nt miRNAs in triggering RDR6-dependent siRNA biogenesis from target transcripts in Arabidopsis. Nat Struct Mol Biol 17: 997–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadami E, Moser M, Zwiebel M, Krczal G, Wassenegger M, Dalakouras A (2013) An endogene-resembling transgene delays the onset of silencing and limits siRNA accumulation. FEBS Lett 587: 706–710 [DOI] [PubMed] [Google Scholar]
- Dadami E, Dalakouras A, Zwiebel M, Krczal G, Wassenegger M (2014) An endogene-resembling transgene is resistant to DNA methylation and systemic silencing. RNA Biol 11: 934–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalakouras A, Dadami E, Wassenegger M (2015) Engineering viroid resistance. Viruses 7: 634–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalakouras A, Wassenegger M, McMillan JN, Cardoza V, Maegele I, Dadami E, Runne M, Krczal G, Wassenegger M (2016) Induction of silencing in plants by high-pressure spraying of in vitro-synthesized small RNAs. Front Plant Sci 7: 1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalakouras A, Jarausch W, Buchholz G, Bassler A, Braun M, Manthey T, Krczal G, Wassenegger M (2018) Delivery of hairpin RNAs and small RNAs into woody and herbaceous plants by trunk injection and petiole absorption. Front Plant Sci 9: 1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmay T, Hamilton A, Rudd S, Angell S, Baulcombe DC (2000) An RNA-dependent RNA polymerase gene in Arabidopsis is required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell 101: 543–553 [DOI] [PubMed] [Google Scholar]
- Dubrovina AS, Kiselev KV (2019) Exogenous RNAs for gene regulation and plant resistance. Int J Mol Sci 20: E2282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovina AS, Aleynova OA, Kalachev AV, Suprun AR, Ogneva ZV, Kiselev KV (2019) Induction of transgene suppression in plants via external application of synthetic dsRNA. Int J Mol Sci 20: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eamens A, Wang MB, Smith NA, Waterhouse PM (2008) RNA silencing in plants: Yesterday, today, and tomorrow. Plant Physiol 147: 456–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391: 806–811 [DOI] [PubMed] [Google Scholar]
- Flores R, Gago-Zachert S, Serra P, Sanjuán R, Elena SF (2014) Viroids: Survivors from the RNA world? Annu Rev Microbiol 68: 395–414 [DOI] [PubMed] [Google Scholar]
- Flores R, Navarro B, Kovalskaya N, Hammond RW, Di Serio F (2017) Engineering resistance against viroids. Curr Opin Virol 26: 1–7 [DOI] [PubMed] [Google Scholar]
- Gan D, Zhang J, Jiang H, Jiang T, Zhu S, Cheng B (2010) Bacterially expressed dsRNA protects maize against SCMV infection. Plant Cell Rep 29: 1261–1268 [DOI] [PubMed] [Google Scholar]
- Gogoi A, Sarmah N, Kaldis A, Perdikis D, Voloudakis A (2017) Plant insects and mites uptake double-stranded RNA upon its exogenous application on tomato leaves. Planta 246: 1233–1241 [DOI] [PubMed] [Google Scholar]
- Good L, Stach JE (2011) Synthetic RNA silencing in bacteria—antimicrobial discovery and resistance breaking. Front Microbiol 2: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grbić M, Van Leeuwen T, Clark RM, Rombauts S, Rouzé P, Grbić V, Osborne EJ, Dermauw W, Ngoc PC, Ortego F, et al. (2011) The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature 479: 487–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AJ, Baulcombe DC (1999) A species of small antisense RNA in posttranscriptional gene silencing in plants. Science 286: 950–952 [DOI] [PubMed] [Google Scholar]
- Huang G, Allen R, Davis EL, Baum TJ, Hussey RS (2006) Engineering broad root-knot resistance in transgenic plants by RNAi silencing of a conserved and essential root-knot nematode parasitism gene. Proc Natl Acad Sci USA 103: 14302–14306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itaya A, Zhong X, Bundschuh R, Qi Y, Wang Y, Takeda R, Harris AR, Molina C, Nelson RS, Ding B (2007) A structured viroid RNA serves as a substrate for dicer-like cleavage to produce biologically active small RNAs but is resistant to RNA-induced silencing complex-mediated degradation. J Virol 81: 2980–2994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashuta S, Zhang Y, Wiggins BE, Ramaseshadri P, Segers GC, Johnson S, Meyer SE, Kerstetter RA, McNulty BC, Bolognesi R, et al. (2015) Environmental RNAi in herbivorous insects. RNA 21: 840–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay F, Vitel M, Brioudes F, Louis M, Knobloch T, Voinnet O (2019) Chemical enhancers of post-transcriptional gene-silencing in Arabidopsis. RNA doi:10.1261/rna.068627.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Ding L, He B, Shen J, Xu Z, Yin M, Zhang X (2014) Systemic gene silencing in plants triggered by fluorescent nanoparticle-delivered double-stranded RNA. Nanoscale 6: 9965–9969 [DOI] [PubMed] [Google Scholar]
- Joga MR, Zotti MJ, Smagghe G, Christiaens O (2016) RNAi efficiency, systemic properties, and novel delivery methods for pest insect control: What we know so far. Front Physiol 7: 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantidis K, Psaradakis S, Tabler M, Tsagris M (2002) The occurrence of CMV-specific short RNAs in transgenic tobacco expressing virus-derived double-stranded RNA is indicative of resistance to the virus. Mol Plant Microbe Interact 15: 826–833 [DOI] [PubMed] [Google Scholar]
- Kalantidis K, Tsagris M, Tabler M (2006) Spontaneous short-range silencing of a GFP transgene in Nicotiana benthamiana is possibly mediated by small quantities of siRNA that do not trigger systemic silencing. Plant J 45: 1006–1016 [DOI] [PubMed] [Google Scholar]
- Kalantidis K, Schumacher HT, Alexiadis T, Helm JM (2008) RNA silencing movement in plants. Biol Cell 100: 13–26 [DOI] [PubMed] [Google Scholar]
- Kaldis A, Berbati M, Melita O, Reppa C, Holeva M, Otten P, Voloudakis A (2018) Exogenously applied dsRNA molecules deriving from the Zucchini yellow mosaic virus (ZYMV) genome move systemically and protect cucurbits against ZYMV. Mol Plant Pathol 19: 883–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid A, Zhang Q, Yasir M, Li F (2017) Small RNA based genetic engineering for plant viral resistance: Application in crop protection. Front Microbiol 8: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim VN. (2008) Sorting out small RNAs. Cell 133: 25–26 [DOI] [PubMed] [Google Scholar]
- Koch A, Kogel KH (2014) New wind in the sails: Improving the agronomic value of crop plants through RNAi-mediated gene silencing. Plant Biotechnol J 12: 821–831 [DOI] [PubMed] [Google Scholar]
- Koch A, Biedenkopf D, Furch A, Weber L, Rossbach O, Abdellatef E, Linicus L, Johannsmeier J, Jelonek L, Goesmann A, et al. (2016) An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Pathog 12: e1005901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konakalla NC, Kaldis A, Berbati M, Masarapu H, Voloudakis AE (2016) Exogenous application of double-stranded RNA molecules from TMV p126 and CP genes confers resistance against TMV in tobacco. Planta 244: 961–969 [DOI] [PubMed] [Google Scholar]
- Kwon DH, Park JH, Ashok PA, Lee U, Lee SH (2016) Screening of target genes for RNAi in Tetranychus urticae and RNAi toxicity enhancement by chimeric genes. Pestic Biochem Physiol 130: 1–7 [DOI] [PubMed] [Google Scholar]
- Lau S, Mazumdari P, Hee T, Song A, Othma R, Harikrishna J (2014) Crude extracts of bacterially-expressed dsRNA protect orchid plants against Cymbidium mosaic virus during transplantation from in vitro culture. J Hortic Sci Biotechnol 89: 569–576 [Google Scholar]
- Lau SE, Schwarzacher T, Othman RY, Harikrishna JA (2015) dsRNA silencing of an R2R3-MYB transcription factor affects flower cell shape in a Dendrobium hybrid. BMC Plant Biol 15: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Niu JZ, Zotti M, Sun QZ, Zhu L, Zhang J, Liao CY, Dou W, Wei DD, Wang JJ, et al. (2017) Characterization and expression patterns of key ecdysteroid biosynthesis and signaling genes in a spider mite (Panonychus citri). Insect Biochem Mol Biol 87: 136–146 [DOI] [PubMed] [Google Scholar]
- Li H, Guan R, Guo H, Miao X (2015) New insights into an RNAi approach for plant defence against piercing-sucking and stem-borer insect pests. Plant Cell Environ 38: 2277–2285 [DOI] [PubMed] [Google Scholar]
- Lindroth AM, Cao X, Jackson JP, Zilberman D, McCallum CM, Henikoff S, Jacobsen SE (2001) Requirement of CHROMOMETHYLASE3 for maintenance of CpXpG methylation. Science 292: 2077–2080 [DOI] [PubMed] [Google Scholar]
- Liu Q, Feng Y, Zhu Z (2009) Dicer-like (DCL) proteins in plants. Funct Integr Genomics 9: 277–286 [DOI] [PubMed] [Google Scholar]
- Lomate PR, Bonning BC (2016) Distinct properties of proteases and nucleases in the gut, salivary gland and saliva of southern green stink bug, Nezara viridula. Sci Rep 6: 27587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Z, Chen Z (2007) Improperly terminated, unpolyadenylated mRNA of sense transgenes is targeted by RDR6-mediated RNA silencing in Arabidopsis. Plant Cell 19: 943–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majidiani S, PourAbad R, Laudani F, Campolo C, Zappalà L, Rahmani S, Mohammadi S, Palmeri P (2019) RNAi in Tuta absoluta management: Effects of injection and root delivery of dsRNAs. J Pest Sci 92: 1409–1419 [Google Scholar]
- Manavella PA, Koenig D, Weigel D (2012) Plant secondary siRNA production determined by microRNA-duplex structure. Proc Natl Acad Sci USA 109: 2461–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez de Alba AE, Elvira-Matelot E, Vaucheret H (2013) Gene silencing in plants: A diversity of pathways. Biochim Biophys Acta 1829: 1300–1308 [DOI] [PubMed] [Google Scholar]
- McHale M, Eamens AL, Finnegan EJ, Waterhouse PM (2013) A 22-nt artificial microRNA mediates widespread RNA silencing in Arabidopsis. Plant J 76: 519–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin AG, Wytinck N, Walker PL, Girard IJ, Rashid KY, de Kievit T, Fernando WGD, Whyard S, Belmonte MF (2018) Identification and application of exogenous dsRNA confers plant protection against Sclerotinia sclerotiorum and Botrytis cinerea. Sci Rep 8: 7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellbye BL, Puckett SE, Tilley LD, Iversen PL, Geller BL (2009) Variations in amino acid composition of antisense peptide-phosphorodiamidate morpholino oligomer affect potency against Escherichia coli in vitro and in vivo. Antimicrob Agents Chemother 53: 525–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mette MF, van der Winden J, Matzke MA, Matzke AJ (1999) Production of aberrant promoter transcripts contributes to methylation and silencing of unlinked homologous promoters in trans. EMBO J 18: 241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S, Cai T, Hu Y, Chen Y, Hodges E, Ni F, Wu L, Li S, Zhou H, Long C, et al. (2008) Sorting of small RNAs into Arabidopsis Argonaute complexes is directed by the 5′ terminal nucleotide. Cell 133: 116–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitter N, Worrall EA, Robinson KE, Li P, Jain RG, Taochy C, Fletcher SJ, Carroll BJ, Lu GQ, Xu ZP (2017) Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat Plants 3: 16207. [DOI] [PubMed] [Google Scholar]
- Mlotshwa S, Pruss GJ, Peragine A, Endres MW, Li J, Chen X, Poethig RS, Bowman LH, Vance V (2008) DICER-LIKE2 plays a primary role in transitive silencing of transgenes in Arabidopsis. PLoS One 3: e1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehl A, Soininen M, Poranen MM, Heinlein M (2018) Synthetic biology approach for plant protection using dsRNA. Plant Biotechnol J 16: 1679–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu J, Shen G, Christiaens O, Smagghe G, He L, Wang J (2018) Beyond insects: Current status and achievements of RNA interference in mite pests and future perspectives. Pest Manag Sci 74: 2680–2687 [DOI] [PubMed] [Google Scholar]
- Nowara D, Gay A, Lacomme C, Shaw J, Ridout C, Douchkov D, Hensel G, Kumlehn J, Schweizer P (2010) HIGS: Host-induced gene silencing in the obligate biotrophic fungal pathogen Blumeria graminis. Plant Cell 22: 3130–3141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numata K, Ohtani M, Yoshizumi T, Demura T, Kodama Y (2014) Local gene silencing in plants via synthetic dsRNA and carrier peptide. Plant Biotechnol J 12: 1027–1034 [DOI] [PubMed] [Google Scholar]
- Overall LM, Rebek EJ (2015) Seasonal abundance and natural inoculativity of insect vectors of Xylella fastidiosa in Oklahoma tree nurseries and vineyards. J Econ Entomol 108: 2536–2545 [DOI] [PubMed] [Google Scholar]
- Palauqui JC, Vaucheret H (1998) Transgenes are dispensable for the RNA degradation step of cosuppression. Proc Natl Acad Sci USA 95: 9675–9680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JS, Bouteiller N, Elmayan T, Vaucheret H (2015) Respective contributions of Arabidopsis DCL2 and DCL4 to RNA silencing. Plant J 81: 223–232 [DOI] [PubMed] [Google Scholar]
- Pélissier T, Thalmeir S, Kempe D, Sänger HL, Wassenegger M (1999) Heavy de novo methylation at symmetrical and non-symmetrical sites is a hallmark of RNA-directed DNA methylation. Nucleic Acids Res 27: 1625–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontes O, Vitins A, Ream TS, Hong E, Pikaard CS, Costa-Nunes P (2013) Intersection of small RNA pathways in Arabidopsis thaliana sub-nuclear domains. PLoS One 8: e65652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooggin MM. (2017) RNAi-mediated resistance to viruses: A critical assessment of methodologies. Curr Opin Virol 26: 28–35 [DOI] [PubMed] [Google Scholar]
- Prins M, Laimer M, Noris E, Schubert J, Wassenegger M, Tepfer M (2008) Strategies for antiviral resistance in transgenic plants. Mol Plant Pathol 9: 73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumplin N, Sarazin A, Jullien PE, Bologna NG, Oberlin S, Voinnet O (2016) DNA methylation influences the expression of DICER-LIKE4 isoforms, which encode proteins of alternative localization and function. Plant Cell 28: 2786–2804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi T, Guo J, Peng H, Liu P, Kang Z, Guo J (2019) Host-induced gene silencing: A powerful strategy to control diseases of wheat and barley. Int J Mol Sci 20: E206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa C, Kamita SG, Dequine H, Wuriyanghan H, Lindbo JA, Falk BW (2010) RNAi effects on actin mRNAs in Homalodisca vitripennis cells. J RNAi Gene Silencing 6: 361–366 [PMC free article] [PubMed] [Google Scholar]
- Rosa C, Kuo YW, Wuriyanghan H, Falk BW (2018) RNA interference mechanisms and applications in plant pathology. Annu Rev Phytopathol 56: 581–610 [DOI] [PubMed] [Google Scholar]
- Ruiz-Ferrer V, Voinnet O (2009) Roles of plant small RNAs in biotic stress responses. Annu Rev Plant Biol 60: 485–510 [DOI] [PubMed] [Google Scholar]
- Safarova D, Brazda P, Navratil M (2014) Effect of artificial dsRNA on infection of pea plants by pea seed-borne mosaic virus. Czech J Genet Plant Breed 50: 105–108 [Google Scholar]
- Sammons R, Ivashuta S, Liu H, Wang D, Feng P, Kouranov A, Andersen S (September 15, 2011) Polynucleotide molecules for gene regulation in plants. US Patent Applicaton No. US20110296556A1
- San Miguel K, Scott JG (2016) The next generation of insecticides: DsRNA is stable as a foliar-applied insecticide. Pest Manag Sci 72: 801–809 [DOI] [PubMed] [Google Scholar]
- Schwach F, Vaistij FE, Jones L, Baulcombe DC (2005) An RNA-dependent RNA polymerase prevents meristem invasion by potato virus X and is required for the activity but not the production of a systemic silencing signal. Plant Physiol 138: 1842–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwind N, Zwiebel M, Itaya A, Ding B, Wang MB, Krczal G, Wassenegger M (2009) RNAi-mediated resistance to Potato spindle tuber viroid in transgenic tomato expressing a viroid hairpin RNA construct. Mol Plant Pathol 10: 459–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Yang G, Chen Y, Yan P, Tuo D, Li X, Zhou P (2014) Resistance of non-transgenic papaya plants to papaya ringspot virus (PRSV) mediated by intron-containing hairpin dsRNAs expressed in bacteria. Acta Virol 58: 261–266 [DOI] [PubMed] [Google Scholar]
- Sherman J, Munyikwa T, Chan S, Petrick J, Witwer K, Choudhuri S (2015) RNAi technologies in agricultural biotechnology: The Toxicology Forum 40th Annual Summer Meeting. Regul Toxicol Pharmacol 73: 671–680 [DOI] [PubMed] [Google Scholar]
- Shukla JN, Kalsi M, Sethi A, Narva KE, Fishilevich E, Singh S, Mogilicherla K, Palli SR (2016) Reduced stability and intracellular transport of dsRNA contribute to poor RNAi response in lepidopteran insects. RNA Biol 13: 656–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy D, Burgyán J (2004) Effects and side-effects of viral RNA silencing suppressors on short RNAs. Trends Plant Sci 9: 76–83 [DOI] [PubMed] [Google Scholar]
- Song XS, Gu KX, Duan XX, Xiao XM, Hou YP, Duan YB, Wang JX, Yu N, Zhou MG (2018) Secondary amplification of siRNA machinery limits the application of spray-induced gene silencing. Mol Plant Pathol 19: 2543–2560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Nunes MA, España MU, Namin HH, Jin P, Bensoussan N, Zhurov V, Rahman T, De Clercq R, Hilson P, et al. (2017) RNAi-based reverse genetics in the chelicerate model Tetranychus urticae: A comparative analysis of five methods for gene silencing. PLoS One 12: e0180654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szittya G, Silhavy D, Molnár A, Havelda Z, Lovas A, Lakatos L, Bánfalvi Z, Burgyán J (2003) Low temperature inhibits RNA silencing-mediated defence by the control of siRNA generation. EMBO J 22: 633–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabler M, Tsagris M (2004) Viroids: Petite RNA pathogens with distinguished talents. Trends Plant Sci 9: 339–348 [DOI] [PubMed] [Google Scholar]
- Tenllado F, Díaz-Ruíz JR (2001) Double-stranded RNA-mediated interference with plant virus infection. J Virol 75: 12288–12297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenllado F, Martínez-García B, Vargas M, Díaz-Ruíz JR (2003) Crude extracts of bacterially expressed dsRNA can be used to protect plants against virus infections. BMC Biotechnol 3: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenllado F, Llave C, Díaz-Ruíz JR (2004) RNA interference as a new biotechnological tool for the control of virus diseases in plants. Virus Res 102: 85–96 [DOI] [PubMed] [Google Scholar]
- Tsagris EM, Martínez de Alba AE, Gozmanova M, Kalantidis K (2008) Viroids. Cell Microbiol 10: 2168–2179 [DOI] [PubMed] [Google Scholar]
- Vélez AM, Fishilevich E (2018) The mysteries of insect RNAi: A focus on dsRNA uptake and transport. Pestic Biochem Physiol 151: 25–31 [DOI] [PubMed] [Google Scholar]
- Voinnet O, Baulcombe DC (1997) Systemic signalling in gene silencing. Nature 389: 553. [DOI] [PubMed] [Google Scholar]
- Wakasa Y, Kawakatsu T, Harada T, Takaiwa F (2018) Transgene-independent heredity of RdDM-mediated transcriptional gene silencing of endogenous genes in rice. Plant Biotechnol J 16: 2007–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Beyene G, Zhai J, Feng S, Fahlgren N, Taylor NJ, Bart R, Carrington JC, Jacobsen SE, Ausin I (2015) CG gene body DNA methylation changes and evolution of duplicated genes in cassava. Proc Natl Acad Sci USA 112: 13729–13734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Weiberg A, Lin FM, Thomma BP, Huang HD, Jin H (2016) Bidirectional cross-kingdom RNAi and fungal uptake of external RNAs confer plant protection. Nat Plants 2: 16151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassenegger M. (2005) The role of the RNAi machinery in heterochromatin formation. Cell 122: 13–16 [DOI] [PubMed] [Google Scholar]
- Wassenegger M, Heimes S, Riedel L, Sänger HL (1994) RNA-directed de novo methylation of genomic sequences in plants. Cell 76: 567–576 [DOI] [PubMed] [Google Scholar]
- Weiberg A, Wang M, Lin FM, Zhao H, Zhang Z, Kaloshian I, Huang HD, Jin H (2013) Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 342: 118–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrall EA, Bravo-Cazar A, Nilon AT, Fletcher SJ, Robinson KE, Carr JP, Mitter N (2019) Exogenous application of RNAi-inducing double-stranded RNA inhibits aphid-mediated transmission of a plant virus. Front Plant Sci 10: 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Ebright YW, Yu B, Chen X (2006) HEN1 recognizes 21–24 nt small RNA duplexes and deposits a methyl group onto the 2′ OH of the 3′ terminal nucleotide. Nucleic Acids Res 34: 667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin G, Sun Z, Liu N, Zhang L, Song Y, Zhu C, Wen F (2009) Production of double-stranded RNA for interference with TMV infection utilizing a bacterial prokaryotic expression system. Appl Microbiol Biotechnol 84: 323–333 [DOI] [PubMed] [Google Scholar]
- Yin GH, Sun ZN, Song YZ, An HL, Zhu CX, Wen FJ (2010) Bacterially expressed double-stranded RNAs against hot-spot sequences of tobacco mosaic virus or potato virus Y genome have different ability to protect tobacco from viral infection. Appl Biochem Biotechnol 162: 1901–1914 [DOI] [PubMed] [Google Scholar]
- Yoon JS, Sahoo DK, Maiti IB, Palli SR (2018) Identification of target genes for RNAi-mediated control of the Two-Spotted Spider Mite. Sci Rep 8: 14687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Yang Z, Li J, Minakhina S, Yang M, Padgett RW, Steward R, Chen X (2005) Methylation as a crucial step in plant microRNA biogenesis. Science 307: 932–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Demirer GS, Zhang H, Ye T, Goh NS, Aditham AJ, Cunningham FJ, Fan C, Landry MP (2019) DNA nanostructures coordinate gene silencing in mature plants. Proc Natl Acad Sci USA 116: 7543–7548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotti MJ, Smagghe G (2015) RNAi technology for insect management and protection of beneficial insects from diseases: Lessons, challenges and risk assessments. Neotrop Entomol 44: 197–213 [DOI] [PubMed] [Google Scholar]
- Zotti M, Dos Santos EA, Cagliari D, Christiaens O, Taning CNT, Smagghe G (2018) RNA interference technology in crop protection against arthropod pests, pathogens and nematodes. Pest Manag Sci 74: 1239–1250 [DOI] [PubMed] [Google Scholar]