Fig. 1.
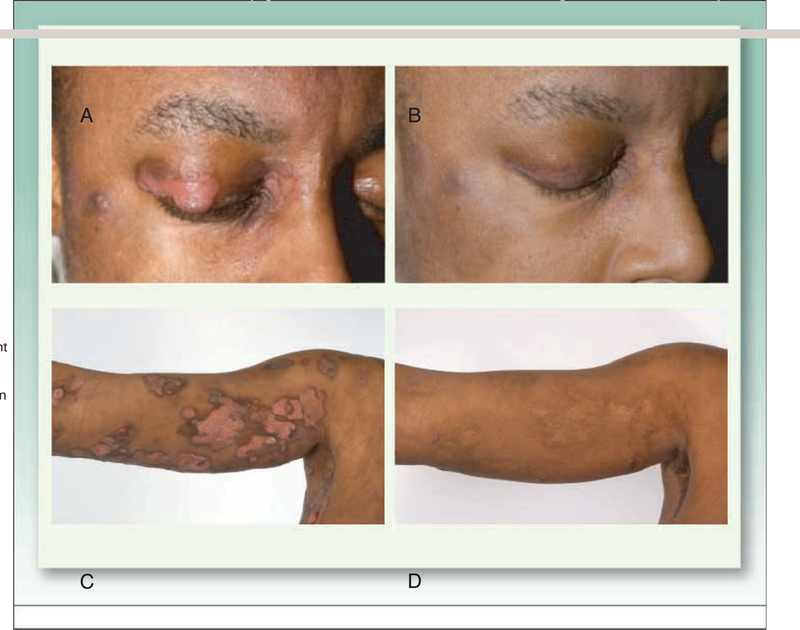
This patient with tumor stage CTCL was enrolled on a phase II trial of romidepsin for patients with CTCL or PTCL and treated at 14 mg/m2 on a day 1,8, and 15 schedule every 28 d (50). A, As shown in the upper panel,a lesion next to the eye and on the eyelid showed significant improvement, B, after the first cycle of therapy. Lesions in other areas of the body also showed significant response,as shown in the photograph, C, of her right arm prior to and, D, 5 mo after starting therapy.This partial response lasted 15 mo.