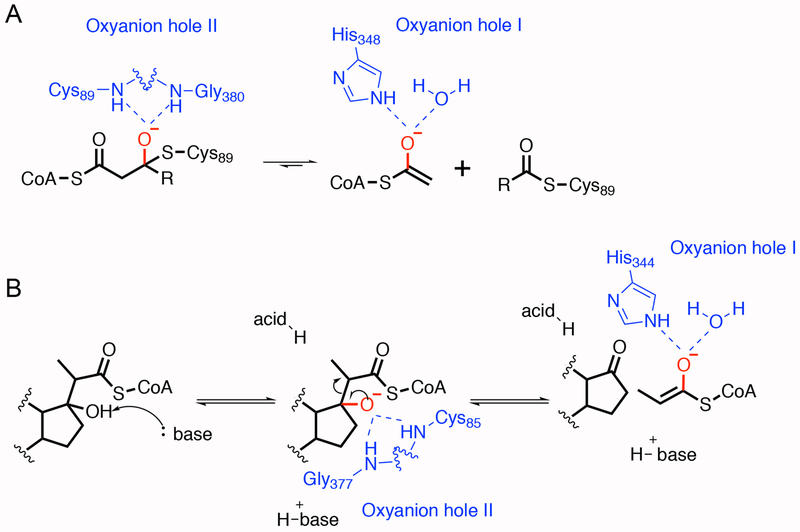
Figure 10. Oxyanion holes in the Zoogloea ramigera biosynthetic acyl-CoA thiolase and the proposed catalytic mechanism of Ltp2.
A. In the Zoogloea ramigera biosynthetic acyl-CoA thiolase, oxyanion hole I, which consists of the acid-base histidine and a water molecule, stabilizes the enolate intermediate. Oxyanion hole II is formed by the nucleophilic cysteine and a glycine residue. It stabilizes the tetrahedral intermediate.24 B. For the retro-aldol reaction catalyzed by Ltp2, a base residue first deprotonates the hydroxyl group at C17 with the stabilization of the oxyanion in hole I. The steroid oxide anion then undergoes elimination to release one molecule of propionyl-CoA. The proton donor in this system is not defined. The proton could come from a water molecule or other residues in the substrate binding pocket.