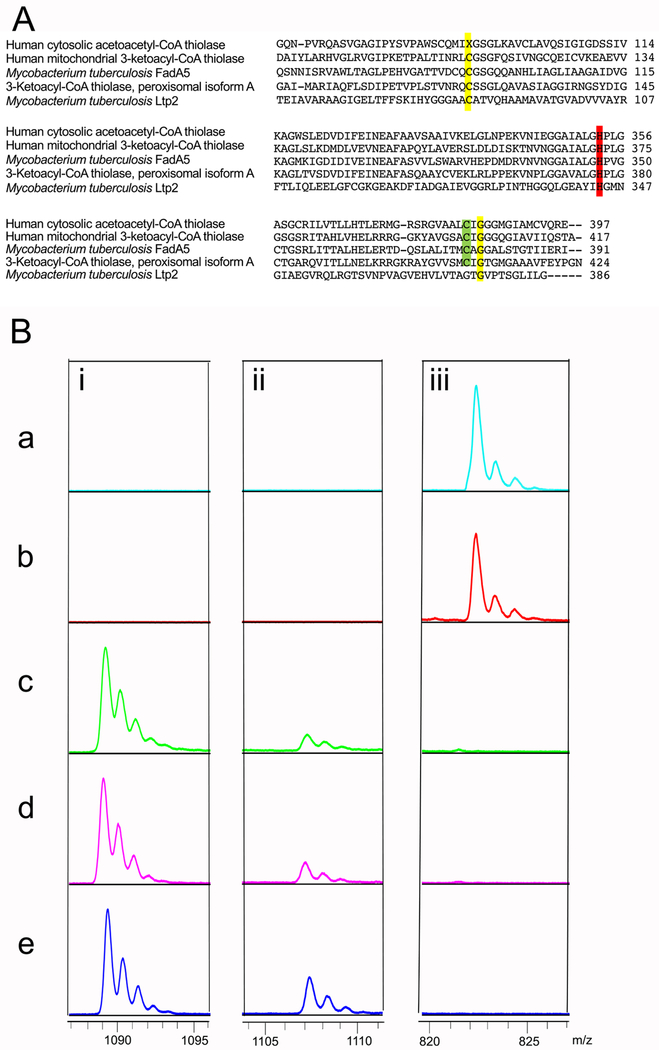
Figure 7. Ltp2 His344 is required for formation of propionyl-CoA from 3-OPDC-CoA.
(A) Sequence alignment of Ltp2 active site with thiolase active sites. Key catalytic residues are highlighted. The alignment was performed with ClustalW2. (B) Analysis of product formation catalyzed by 10 μM wild-type or mutant enzyme (a) ChsH1-ChsH2-Ltp2, (b) ChsH1-ChsH2-Ltp2C85A, (c) ChsH1-ChsH2-Ltp2H344A, (d) ChsH1-ChsH2-Ltp2C85A,H344A, and (e) ChsH1-ChsH2 with 3-OPDC-CoA (100 M) as substrate at 25 °C. Reactions were analyzed by MALDI-TOF mass spectrometry analysis in negative ionization mode, the peaks at i. 1089.2 m/z [M-H]−, ii: 1107.2 m/z [M-H]−, and iii: 822.4 m/z [M-H]-correspond to 3-OPDC-CoA, 17-HOPC-CoA, and propionyl-CoA, respectively.