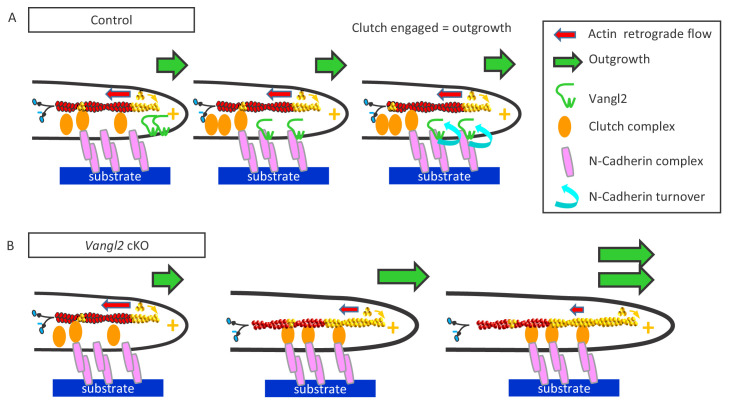
Figure 9. Vangl2 inhibits neuronal outgrowth by regulating N-cadherin engagement in the clutch mechanism.
(A) The retrograde actin flow is generated by constant polymerization (at the tip of filopodia/lamellipodia) and depolymerization (minus end) of actin monomers along a filament (F-actin), and participates to the extension and retraction of filopodia/lamellipodia. When permissive substrates such as N-cadherin or laminin are present in the environment they bind to corresponding cell surface receptors at the plasma membrane, which recruit specific adhesion complexes, creating a molecular link between the adhesion proteins and F-actin filaments within the growth cone. This ‘molecular clutch’ leads to the local engagement of the actin flow, causing a decrease in its speed and a proportional increase in neuronal outgrowth, due to the ongoing actin polymerization. The presence of Vangl2 at the membrane participates in the regulation of this outgrowth by controlling the number of N-cadherin molecules engaged in the clutch. If no permissive substrate is present, the link is absent, and the outgrowth is slow, while the retrograde flow is fast. (B) The absence of Vangl2 leads to an increased presence/stabilization of N-cadherin at the plasma membrane, and the engagement of more N-cadherin molecules which in turn increase the molecular clutch strength. As a result, a decreased actin retrograde flow speed and an increased neuronal outgrowth are observed.