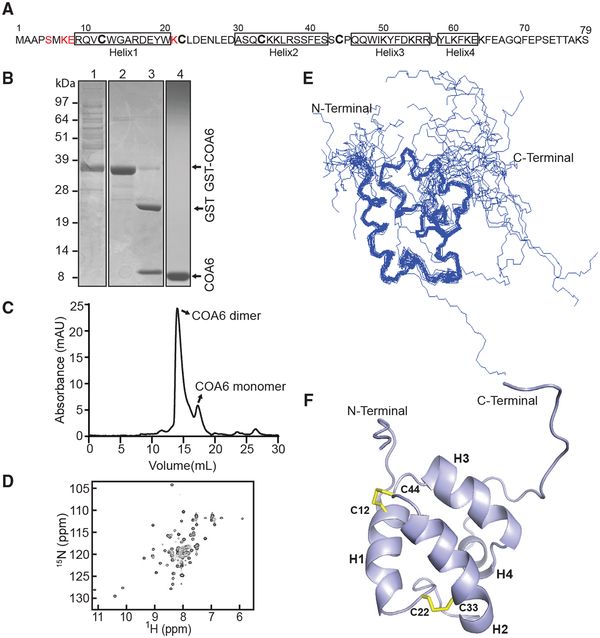
Figure 1. Solution Structure of Human COA6.
(A) Primary structure of human COA6. No NMR assignments were achieved for the residues colored in red.
(B) SDS-PAGE analysis of the samples obtained during COA6 purification steps: lane 1, supernatant of lysate from E. coli expressing the COA6-GST fusion protein; lane 2, eluate containing COA6-GST from the GST binding column; lane 3, COA6-GST after cleaving the GST tag with thrombin; and lane 4, purified COA6 sample from gel filtration chromatography.
(C) Elution profile of purified COA6 from the Superdex 75 gel-filtration column.
(D) 1H-15N HSQC spectrum of human COA6.
(E) Ensemble of the 20 NMR solution structures of COA6 by backbone representation.
(F) Ribbon representation of human COA6 based on the lowest energy conformer of solution structures. Human COA6 has four helixes labeled as H1, H2, H3, and H4, and the two disulfide bonds between H1 and H2 are shown in yellow.